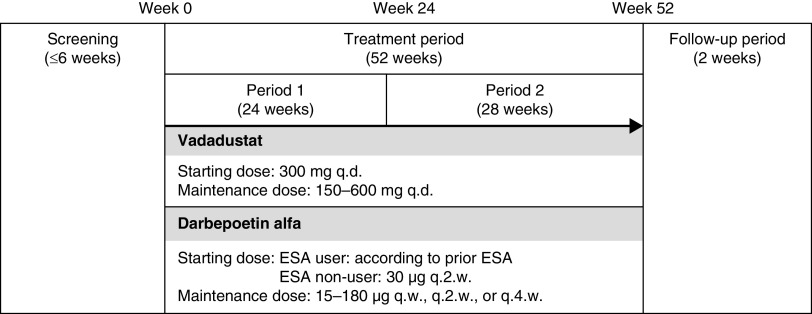
Figure 1.
Study design. The study comprised a screening period, a 52-week treatment period, and a follow-up period. Patients were randomized 1:1 to oral vadadustat or subcutaneous darbepoetin alfa. Vadadustat was started at 300 mg once daily and doses were adjusted (dose range: 150–600 mg once daily) to maintain hemoglobin levels within the predefined target of 11.0–13.0 g/dl. The initial dose of darbepoetin alfa was set in accordance with previous ESAs in ESA users and was 30 μg every 2 weeks in ESA non–users. Doses of darbepoetin alfa were adjusted between 15 and 180 μg once weekly, every 2 weeks, or every 4 weeks to maintain Hb levels within the target range. The primary efficacy end point was the average Hb at weeks 20 and 24. q.d., once daily; q.w., once weekly; q.2.w, every 2 weeks; q.4.w, every 4 weeks