Figure 5.
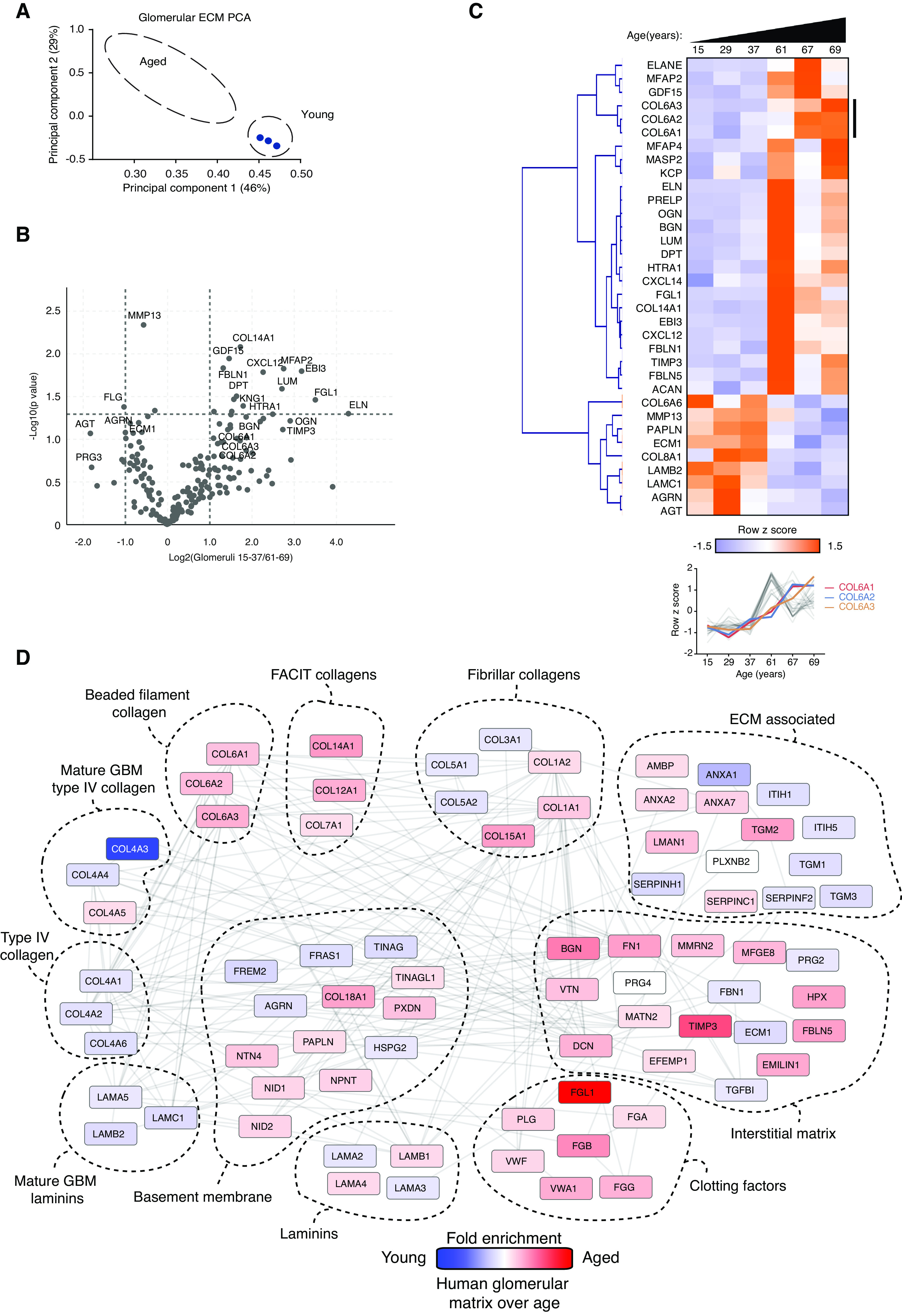
Human aging is associated with marked alterations in glomerular matrix composition. Glomeruli were isolated from young (15, 29, 37 years) and aged (61, 67, 69 years) human kidneys, subjected to matrix fractionation, and analyzed by mass spectrometry. (A) MATLAB was used for PCA of matrisome proteins. Components 1 and 2 are presented and the percentage variance explained by each component is indicated on the relevant axis. (B) Volcano plots of proteins detected in matrix fractions demonstrate differential protein abundance shown as the log2(fold change) of aged over young glomeruli on the x axis and −log10(P value) on the y axis. (C) Unsupervised hierarchical clustered heat map displaying ECM proteins detected in young and aged glomerular datasets. Data were standardized by row z-score and grouped in MeV using Pearson correlation complete-linkage clustering. Inset line graph shows increased type VI collagen protein abundance with age. (D) Protein-protein interaction network of altered human glomerular matrisome proteins. Mass spectrometry data from matrix-enriched protein fractions were filtered for known matrisome proteins. These proteins were mapped onto a merged human, mouse, and rat interactome. Nodes represent proteins and the edges represent reported protein-protein interactions. Color represents fold enrichment to datasets, with increased abundance in aged human glomerular samples illustrated in red and increased abundance in young human glomerular samples illustrated in blue. Proteins are grouped by function.
