Fig. 7. Proposed mechanisms of Treg enrichment and differentiation in αvβ8-expressing tumors.
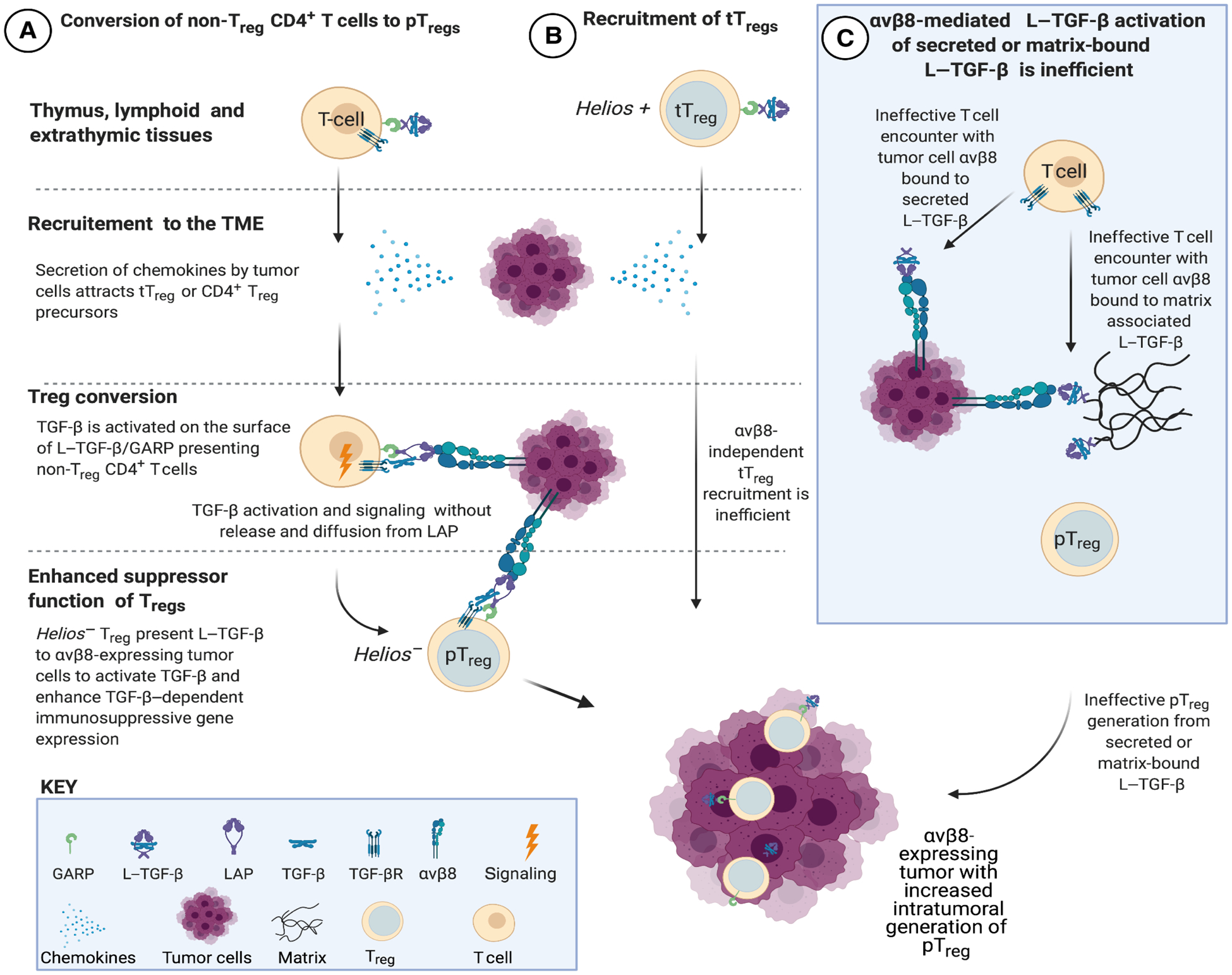
(A) Non-Treg CD4+ T cells expressing L–TGF-β/GARP infiltrate tumors in response to chemokines in the TME (21). TGF-β cannot interact with TGF-βR unless it undergoes activation. L–TGF-β/GARP–expressing non-Treg CD4+ T cells undergo Treg conversion to Helios− pTreg after binding to integrin αvβ8 expressed by tumor cells. The mechanism of TGF-β activation does not require the release and diffusion of TGF-β, ensuring that only T cells in contact with αvβ8-expressing tumor cells are converted to pTreg (29). (B) Thymically derived Helios+ Treg (tTreg) can potentially be recruited to the TME, but this is not evident in αvβ8-expressing tumors. (C) When L–TGF-β is soluble or matrix bound, TGF-βRs are not positioned on the same surface as L–TGF-β. Thus, if active TGF-β is not released from L–TGF-β when exposed to αvβ8 bearing tumor cells, then TGF-βR–expressing T cells need to find, orient, and overcome steric hindrance to bind to TGF-β exposed within the L–TGF-β complex. This activation process is less efficient than when L–TGF-β and TGF-βRs are on the same surface (29). Therefore, soluble or matrix-bound L–TGF-β is less likely to significantly contribute to αvβ8-mediated pTreg conversion in the TME. Created in BioRender.
