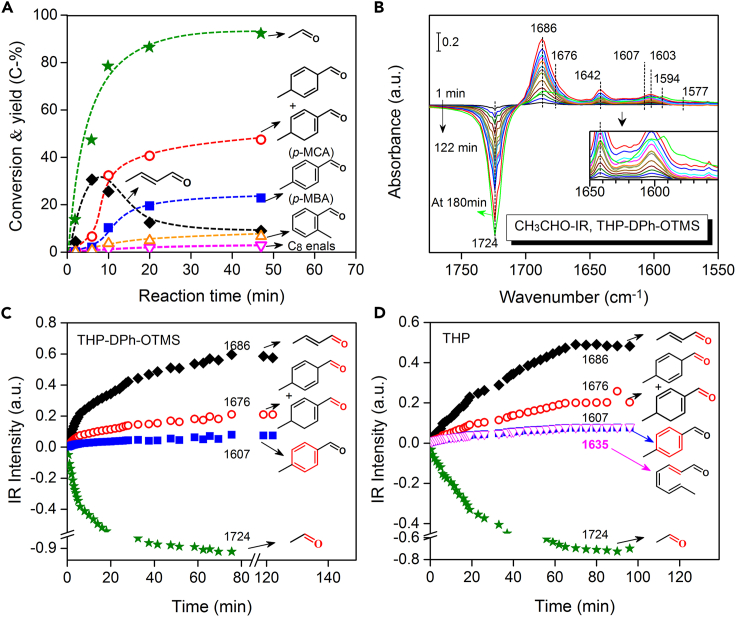
Figure 2.
Reaction route
(A) Dependence of conversion and product yields on the reaction time (1 mmol CH3CHO, 3 mL CHCl3, 0.05 mmol THP-DPh-OTMS, 0.3 mmol p-NO2-PhOH, 20°C).
(B) IR difference spectra. The green line in Figure 2B was obtained at 180 min (45 μmol CH3CHO, 0.5 μmol THP-DPh-OTMS, 1.2 μmol p-NO2-PhOH, CHCl3 as solvent, 20°C).
(C) Intensity changes of IR peaks in Figure 2B with reaction time.
(D) Evolution of IR peaks using THP as a catalyst with reaction time (see Figure S1). Star: CH3CHO, diamond: 2-butenal, hollow cycle: p-MCA, square: p-MBA, hollow up triangle: o-MBA, hollow down triangle: C8 enals.