INTRODUCTION
Since the novel severe acute respiratory syndrome‐coronavirus‐2 (SARS‐CoV‐2) emerged in Wuhan, China, coronavirus disease 2019 (COVID‐19) has rapidly spread worldwide leading to the current pandemic. 1 Olfactory and taste dysfunction (OD, TD) have been included among the most frequent reported symptoms, with a prevalence reported to be 47.85%. 2
Studies published on COVID‐19–related OD have mainly assessed smell loss using patient‐reported outcome measures (PROMs) such as the visual analogue scale (VAS) and the 22‐item Sino‐Nasal Outcome Test (SNOT‐22). 1 However, self‐reported OD poorly correlates with olfactory tests such as Sniffin’ Sticks (S'S). 3
The aim of this study is to provide a prospective long‐term assessment of COVID‐19–related OD using PROMs 4 and S'S 5 and to investigate their correlation.
METHODS
Patients with laboratory‐confirmed SARS‐CoV‐2 infection and OD/TD were selected from our Infectious Disease Department database and asked to complete the SNOT‐22 and VAS for smell and taste (sVAS, tVAS: 0 represents “absent” and 10 “not affected”). Exclusion criteria included previous history of OD/TD, head and neck tumors, chemo/radiotherapy, head trauma, chronic rhinosinusitis and neurologic diseases. The study was approved by the Hospital Research Ethics Committee Protocol 056881.
After disease recovery, patients who completed the initial screening (T0) were invited to undergo S'S 5 evaluation (T1), regardless of their reported olfactory function. SNOT‐22 and s/tVAS were repeated. Patients with a confirmed OD at T1 S'S had a second evaluation (T2) roughly 6 months after their first assessment.
Paired t‐test was used for statistical analysis except for sVAS and tVAS, for which the exact Wilcoxon signed rank test was used. Spearman correlation coefficient was chosen to measure the relationship between the different indicators.
RESULTS
A cohort of 101 consecutive COVID‐19 subjects complaining of chemosensory alteration completed the s/tVAS and the SNOT‐22 within one week of COVID‐19 diagnosis (T0) (Table 1).
TABLE 1.
PROMs, Sniffin’ Sticks scores, 10 and percentage of normosmic, hyposmic, and anosmic patients at T0, T1, and T2
| Parameter | T0 (n = 101) | T1 (n = 83) | T2 (n = 22) | p T0 versus T1 a | p T1 versus T2 a |
|---|---|---|---|---|---|
| sVAS, mean ± SD | 2.33 ± 3.18 | 6.35 ± 3.05 | 7.20 ± 2.71 | <0.0001 | 0.0394 |
| tVAS, mean ± SD | 3.31 ± 3.46 | 7.39 ± 2.63 | 8.30 ± 1.90 | <0.0001 | 0.1614 |
| SNOT‐22, mean ± SD | 41.73 ± 18.24 | 16.12 ± 13.86 | 15.09 ± 11.74 | <0.0001 | 0.1262 |
| NS SNOT‐22, mean ± SD | 7.62 ± 5.46 | 2.76 ± 4.06 | 2.60 ± 3.99 | <0.0001 | 0.1819 |
| Threshold (T), mean ± SD | – | 5.90 ± 3.10 | 5.68 ± 2.71 | – | 0.0557 |
| Discrimination (D), mean ± SD | – | 11.20 ± 2.90 | 12.18 ± 1.53 | – | 0.0009 |
| Identification (I), mean ± SD | – | 11.50 ± 2.40 | 12.59 ± 1.44 | – | 0.0034 |
| TDI score, mean ± SD | – | 28.50 ± 6.50 | 30.34 ± 4.27 | – | <0.0001 |
| Normosmic, n (%) | – | 36 (43.4) | 11 (50.0) | – | – |
| Hyposmic, n (%) | – | 45 (54.2) | 11 (50.0) | – | – |
| Anosmic, n (%) | – | 2 (2.4) | 0 (0.0) | – | – |
Notes: sVAS/tVAS score range: 0 = the worst thinkable situation, 10 = not affected. NS SNOT‐22 items: (1) need to blow nose, (2) sneezing, (3) runny nose, (4) postnasal discharge, (5) thick nasal discharge, and (6) blockage/congestion of nose. TDI score: threshold + discrimination + identification.
Abbreviations: NS SNOT‐22, SNOT‐22 nasal symptoms items without considering those related to smell and taste dysfunctions; PROM, patient‐reported outcome measure; SD, standard deviation; SNOT‐22, 22‐item Sino‐Nasal Outcome Test; VAS, visual analogue scale; sVAS, VAS for smell; tVAS, VAS for taste; T0, subjects’ enrollment; T1, first olfactory evaluation; T2, second olfactory evaluation.
Significant p values are in bold. Level of significance p < 0.05.
Eighty‐one patients underwent further evaluation with S'S at T1 (median time 62 days [range, 41–165 days] from diagnosis). Looking at the individual S'S subscores (threshold, discrimination, and identification [TDI]), the percentage of patients below normal were 44%, 41%, 38%, respectively, with the threshold being the most compromised (Figure 1; Table 1), whereas the composite TDI S'S score was below normal in 55.6% of patients. At T1 both sVAS and tVAS showed a significant improvement when compared to T0 (p < 0.0001, for both) and a statistically significant moderate correlation between sVAS and TDI score was demonstrated (r = 0.42, p = 0.0009). About 55% (25/45) of the S'S hyposmic patients “self‐reported” their olfaction as being recovered, whereas only 72.2% (26/36) of the S'S normosmics reported their smell as normal.
FIGURE 1.
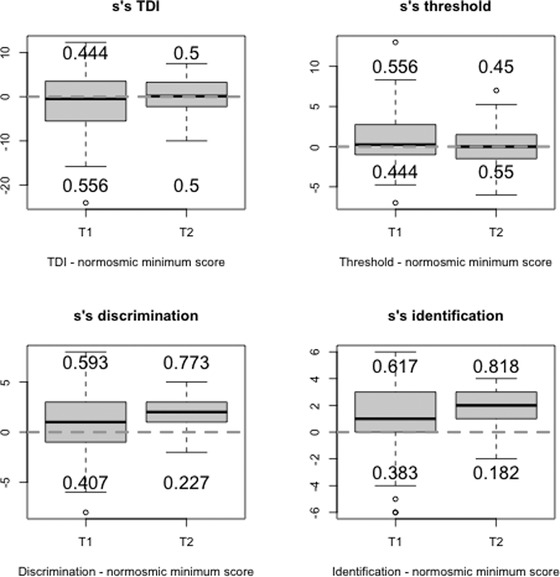
Box‐plots showing the distributions of the differences between patients’ scores (TDI) and the normosmic minimum score (10th percentile of the distribution of the scores for normosmic) 10 at T1 and T2. The numbers indicate the proportion (decimals) of subjects with normal scores (above the dotted line) and with pathological scores (below the dotted line). Dotted line: Normosmic minimum score. Abbreviations: S'S: Sniffin’ Sticks; T1: first psychophysical olfactory test; T2: second psychophysical olfactory test. TDI, threshold, discrimination, and identification.
Twenty‐two patients with a confirmed S'S OD at T1 received a further smell evaluation 6 months later (T2 – median time, 230 days [range, 213–252] from diagnosis). Looking at the S'S subscores separately, only the discrimination and identification scores significantly improved when compared to T1 scores. At T2, only sVAS demonstrated a significant improvement with respect to T1 (p = 0.0394), whereas neither SNOT‐22 nor tVAS changed significantly (Figure 1; Table 1). Similarly, a statistically significant correlation was not found between T2 sVAS and TDI scores (r = 0.15, p = 0.5). At T2, 81.8% of normosmics “self‐reported” their olfaction as recovered, whereas 72.7% of hyposmics reported smell as recovered.
DISCUSSION
Current evidence shows that OD is an early marker of COVID‐19 and one of the best predictors of SARS‐CoV‐2 infection. Our COVID‐19 study provides a prospective long‐term evaluation of OD using both PROMs and S'S. At the first olfactory evaluation (T1), 55.6% of the patients were found to be hypo/anosmic according to the TDI score. Interestingly, when we looked at the S'S subscores separately, we observed that a lower percentage of them showed below‐normal scores. This highlights the importance of subanalysis when evaluating smell function using S'S because the sole use of identification tests for screening may underestimate the real prevalence of olfactory loss. Moreover, we found that at T2 only the discrimination and identification scores improved significantly when compared to T1, indicating that the odor threshold is affected long‐term.
A significant improvement in the self‐reported olfactory and taste loss was shown between T0 and T1, whereas only sVAS improved significantly at T2 when compared to T1. The absence of a T2 tVAS significant improvement could be explained by the T1 tVAS already being at a normal level, suggesting that TD in these patients is not linked to an impairment of gustation itself but to a retronasal impairment. 6
The correlation between self‐rated OD and S'S was moderate (r = 0.42) and significant (p = 0.0009) at T1, but not significant at T2. The lack of correlation observed in the late recovery‐phase could be explained by a subject habituation to OD or to the presence of milder smell impairment, which may not be noticeable by the subject. Because threshold represents the main component being affected long‐term, this would imply the patient's OD lies with their inability to smell odors at low concentration and a potential end‐organ pathogenesis. Our results confirm that psychophysical smell tests remain more sensitive than PROMs 7 and that the latter could be unreliable when used to assess smell recovery in the long‐term. Nevertheless, we recognize that PROMs still remain of value in the evaluation of new‐onset smell loss given their good discriminative ability. 8
The lag between T0 and T1 constitutes a study limitation. Unfortunately, that was mainly related to the patients' need to self‐isolate and demonstrate negative swab tests before coming to the clinic in accordance with Italian guidance.
In conclusion, when assessing olfactory performance in patients with COVID‐19–related OD we discourage the sole use of PROMs and recommend the use of psychophysical tests with additional subtest analysis. We also showed that in COVID‐19–related OD, threshold is the most affected S'S subtest, suggesting an end‐organ failure pathogenesis. 9
CONFLICT OF INTEREST
None of the authors have any conflicts of interest, financial or otherwise.
ACKNOWLEDGMENTS
This work was supported by the Cariparo Foundation grant: “COVID‐19‐CNS understanding neurotropism and long‐term brain damage from COVID‐19.”
Open Access Funding provided by Universita degli Studi di Padova within the CRUI‐CARE Agreement.
Bordin A, Mucignat‐Caretta C, Gaudioso P, et al. Comparison of self‐reported symptoms and psychophysical tests in coronavirus disease 2019 (COVID‐19) subjects experiencing long‐term olfactory dysfunction: a 6‐month follow‐up study. Int Forum Allergy Rhinol. 2021;11:1592–1595. 10.1002/alr.22828.
[Correction added on 16 May 2022, after first online publication: CRUI funding statement has been added.]
REFERENCES
- 1. Marchese‐Ragona R, Restivo DA, De Corso E, et al. Loss of smell in COVID‐19 patients: a critical review with emphasis on the use of olfactory tests. Acta Otorhinolaryngol Ital. 2020;40:241‐247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Saniasiaya J, Islam MA, Abdullah B. Prevalence of olfactory dysfunction in coronavirus disease 2019 (COVID‐19): a meta‐analysis of 27,492 patients. Laryngoscope. 2021;131:865‐878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ta NH, Gao J, Philpott C. A systematic review to examine the relationship between objective and patient‐reported outcome measures in sinonasal disorders: recommendations for use in research and clinical practice. Int Forum Allergy Rhinol. 2021;11:910‐923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ottaviano G, Carecchio M, Scarpa B, Marchese‐Ragona R. Olfactory and rhinological evaluations in SARS‐CoV‐2 patients complaining of olfactory loss. Rhinology. 2020;58:400‐401. [DOI] [PubMed] [Google Scholar]
- 5. Ottaviano G, Cantone E, D'Errico A, et al. Sniffin' Sticks and olfactory system imaging in patients with Kallmann syndrome. Int Forum Allergy Rhinol. 2015;5:855‐861. [DOI] [PubMed] [Google Scholar]
- 6. Pendolino AL, Ottaviano G, Scarpa B, et al. Characteristics of taste dysfunction in COVID‐19 subjects coming from two different countries. J Neurovirol. 2021;22:1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hannum ME, Ramirez VA, Lipson SJ, et al. Objective sensory testing methods reveal a higher prevalence of olfactory loss in COVID‐19‐positive patients compared to subjective methods: a systematic review and meta‐analysis. Chem Senses. 2020;45:865‐874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Prajapati DP, Shahrvini B, MacDonald BV, et al. Association of subjective olfactory dysfunction and 12‐item odor identification testing in ambulatory COVID‐19 patients. Int Forum Allergy Rhinol. 2020;10:1209‐1217. 10.1002/alr.22688. [DOI] [PubMed] [Google Scholar]
- 9. Ottaviano G, Zuccarello D, Frasson G, et al. Olfactory sensitivity and sexual desire in young adult and elderly men: an introductory investigation. Am J Rhinol Allergy. 2013;27:157‐161. [DOI] [PubMed] [Google Scholar]
- 10. Oleszkiewicz A, Schriever VA, Croy I, Hähner A, Hummel T. Updated Sniffin' Sticks normative data based on an extended sample of 9139 subjects. Eur Arch Otorhinolaryngol. 2019;276:719‐728. [DOI] [PMC free article] [PubMed] [Google Scholar]


