Abstract
Due to the known anti‐inflammatory and antiviral effects of zinc, 25(OH)D, and vitamin B12, in this study, we explored the association between serum levels of these micronutrients in coronavirus disease 2019 (COVID‐19) patients at the time of admission and the clinical outcomes. This study was carried out on 293 patients with COVID‐19, who were hospitalized at Imam Hassan hospital (Bojnourd, Iran). We collected demographic data, clinical characteristics, values of serum biochemical parameters in the first week of admission, and clinical outcomes from electronic medical records. We also measured serum levels of zinc, 25(OH)D, and vitamin B12 within 3 days of admission. Of the 293 hospitalized, the median age was 53 years, and 147 (50.17%) were female. Thirty‐seven patients (12.62%) were admitted to the intensive care unit (ICU), and forty‐two (14.32%) died. We found that the serum levels of zinc, vitamin B12, and 25(OH)D were lower in patients who died than those who were admitted to ICU or non‐ICU and survived; however, these differences were not statistically significant for vitamin B12 and 25(OH)D (p > 0.05). The serum concentrations of zinc, vitamin B12, and 25(OH)D at the time of admission did not affect the length of hospital stay in patients with COVID‐19. In general, it seems that serum levels of 25(OH)D, vitamin B12, and especially zinc at the time of admission can affect clinical outcomes in COVID‐19 patients.
Keywords: 25(OH)D, clinical outcome, COVID‐19, vitamin B12, zinc
Highlights
Lower levels of zinc in death cases compared to surviving cases in Covid‐19.
Lower levels of B12 and D in death cases compared to surviving cases in Covid‐19.
No effect of levels of zinc, B12, and D on the length of hospital stay in COVID‐19.
1. INTRODUCTION
The coronavirus disease 2019 (COVID‐19) that is caused by the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has become a clinical threat worldwide. 1 This infection mostly presents with mild flu‐like symptoms, but some patients, especially older individuals and those with underlying illnesses, evolve into critical conditions and rapidly develop acute respiratory distress syndrome, respiratory failure, multiple organ disorder, and death. 2 , 3 The severe forms of the disease are accompanied by an aggressive inflammatory response with the typical characteristics of an uncontrolled systemic inflammatory response resulting from the release of large amounts of pro‐inflammatory cytokines by immune cells known as “cytokine storm.” 4 It has been reported that the severity of COVID‐19 can be influenced by various factors such as age, sex, race, current health issue, and nutritional status. 3 , 5
Micronutrients include vitamins (e.g., A, B, and D) and minerals (e.g., Ca, P, and Mg), as well as trace elements (Zn, CU, and Fe) involved in initiating, maintaining, and regulating host immune reactions to the virus infections through the effect on frequency and function of innate and adaptive immune cells, production and development of virus‐specific antibodies, production of pro‐, and anti‐inflammatory cytokines, oxidative burst reaction, and so forth. 6 , 7
Zinc is an essential micronutrient with potent immunoregulatory and antiviral properties that is involved in the growth and the maturation of immune cells, development and activation of lymphoid cells, regulation of inflammatory cytokines, and controlling oxidative stress. 8 Zinc deficiency is associated with immune system dysfunction, neurosensory disorders, decreased body mass, enhanced risk of inflammatory disorders, increased risk of incidence, and morbidity of viral pneumonia. 7
It is well recognized that vitamin D as an essential mediator of immune responses, has anti‐infective, anti‐inflammatory, and immunomodulant functions. It can modulate immune responses to viral respiratory tract infection and improve lung function through the reduction of inflammation. 6
Vitamin B12 as an immunomodulator can regulate cellular immune responses and can also support hematopoiesis. 9 Reduced number of lymphocytes, suppression of NK cell activity, and decreased CD8+ cells were found in humans with vitamin B12 deficiency. 6 A previous study also showed that vitamin B12 could suppress viral replication in the host cells. 7
It is now well established that insufficiency or deficiency of micronutrients such as serum 25(OH)D, vitamin B12, and zinc can affect host immune responses to viral infections, inflammatory activity, and finally influence clinical outcome in patients with COVID‐19. 10 , 11 , 12 , 13
Although serum levels of zinc, 25(OH)D, and vitamin B12 were investigated in COVID‐19 patients, there is still scientific uncertainty about the relationship between these micronutrients and clinical outcomes in COVID‐19 patients. In other words, can we predict clinical outcomes in COVID‐19 patients through the measurement of serum levels of zinc, 25(OH)D, and vitamin B12 at the time of admission? Therefore, we conducted a study to assess the association between serum levels of micronutrients (including zinc, 25(OH)D, and vitamin B12) at the time of admission and clinical outcomes (e.g., length of stay, intensive care unit [ICU] admission, discharge, and mortality) in COVID‐19 patients.
2. METHODS
2.1. Study design and participants
This single‐center study was carried out on 293 adult patients with COVID‐19 who were hospitalized at Imam Hassan Hospital of North Khorasan University of Medical Sciences (Bojnourd, Iran) from April 20 to August 5, 2020. All the patients who had positive reverse‐transcription polymerase chain reaction tests for SARS‐CoV‐2 and common computed tomography imaging findings associated with COVID‐19 were included in the study. The experimental protocols that have been used in this study were approved by the Ethics Committee of North Khorasan University of Medical Sciences, Bojnourd, Iran (IR.NKUMS.REC.1399.052).
2.2. Data collection and assessment of zinc, 25(OH)D, and vitamin B12 serum levels
We obtained demographic data, exposure history, chronic medical histories, clinical symptoms or signs, clinical outcomes, and hospitalization duration from electronic medical records. We also collected serum samples from polymerase chain reaction‐confirmed COVID‐19 patient samples sent to Imam Hassan hospital laboratory within 3 days of admission to the analysis of serum levels of zinc, 25(OH)D, and vitamin B12.
The serum was analyzed for zinc concentration using commercial kits (ZiestChem). That zinc assay kit is a direct colorimetric assay based on the 5‐Br‐PAPS method without deproteinization of the sample. The reference range used for the zinc concentration was 70–127 μg/dL.
Quantitation of serum 25(OH)D was performed using commercial sandwich enzyme‐linked immunosorbent assay (ELISA) kits (Padtangostar). The intra‐assay and inter‐assay precision for the ELISA were less than 10% and 12% for 25(OH)D. According to current guidelines, vitamin D deficiency is defined if serum 25 (OH)D levels are less than 10 ng/ml, insufficiency as 10–30 ng/ml, and sufficiency as 30–70 ng/ml.
Serum concentrations of vitamin B12 were determined by the sandwich ELISA method and a commercial kit (Monobind). The reference range used for the vitamin B12 concentration was 200–835 pg/ml.
2.3. Statistical analysis
GraphPad Prism software, version 5.0 (GraphPad Software) was used for statistical analysis of the data. The normality of variables (serum levels of zinc, B12, and 25(OH)D) was assessed with the Kolmogorov–Smirnov test. Continuous variables were compared using t‐tests or one‐way analysis of variance, while χ 2 or Fisher exact tests were used for categorical dependent data analysis. Data were expressed as mean ± standard deviation (SD). Values of p < 0.05 (*) were considered significant.
3. RESULTS
3.1. Demographic data and clinical characteristics
Of 293 hospitalized patients with COVID‐19, 147 (50.17%) were female, and 146 (49.83%) were male. The median age was 53 years (interquartile range [IQR]: 38–65). Thirty‐seven of the 293 patients (12.62%) were admitted to the ICU, and 42 (14.32%) died. Approximately 8.5% of patients with COVID‐19 required intubation in the first 24 h of admission. We found significant correlations between age and ICU admission, intubation, or death (p < 0.05).
The most common symptoms of illness onset were cough (60.06%), fever (55.97%), dyspnea (54.26%), myalgia or fatigue (24.91%), diarrhea (15.35%), and headache (8.14%). 137 (46.75%) of 293 patients had underlying comorbid medical disorders, including cardiovascular disease (27.64%), diabetes (16.04%), and chronic kidney diseases (6.4%), and hypertension (6.4%). The demographic data and clinical characteristics of all patients are shown in Table 1.
Table 1.
Demographic data and baseline characteristics in patients with COVID‐19
| Variable | n (%) |
|---|---|
| Age | |
| <60 | 169 (57.67) |
| ≥60 | 124 (42.32) |
| Sex | |
| Female | 147 (50.17) |
| Male | 146 (49.83) |
| Exposure history | 57 (19.45) |
| Length of hospital stay | |
| <7 | 171 (58.36) |
| ≥7 | 122 (41.64) |
| Symptoms and signs at admission | |
| Fever | 164 (55.97) |
| Cough | 176 (60.06) |
| Fatigue or myalgia | 73 (24.91) |
| Nausea and vomiting | 19 (6.48) |
| Diarrhea | 45 (15.35) |
| Headache | 24 (8.19) |
| Required intubation | 25 (8.53) |
| Death | 42 (14.33) |
| Need for ICU | 37 (12.62) |
| Comorbidities | 137 (46.75) |
| Diabetes | 47 (16.04) |
| Cardiovascular disease | 81 (27.64) |
| Chronic kidney disease | 18 (6.14) |
| Hypertension | 18 (6.14) |
| Endocrine system disease | 7 (2.38) |
| Tumor | 4 (1.36) |
Abbreviations: COVID‐19, coronavirus disease 2019; ICU, intensive care unit.
3.2. Serum zinc, vitamin B12, and 25 (OH)D concentrations in patients with COVID‐19
Our results showed that serum zinc levels are significantly lower in patients who died than those who were admitted to ICU or non‐ICU and survived (94.17 ± 25.95 µg/dL vs. 98.83 ± 30.49 µg/dL and 118.8 ± 34.40 µg/dL, p = 0.002). We also observed that patients who died due to COVID‐19 had lower than vitamin B12 compared with those who were admitted to ICU or non‐ICU, but no significant difference among them (404.4 ± 70.18 pg/ml vs. 499.6 ± 82.80 pg/ml and 465.4 ± 35.70 pg/ml, p = 0.78). The measurement of 25(OH)D serum levels exhibited that the concentration of this vitamin in the death group (21.48 ± 11.71 ng/ml) is lower than ICU (29.02 ± 19.32 ng/ml) and non‐ICU (29.70 ± 20.27 ng/ml) admission groups, although these differences were not statistically significant (p = 0.21) (Figure 1).
Figure 1.
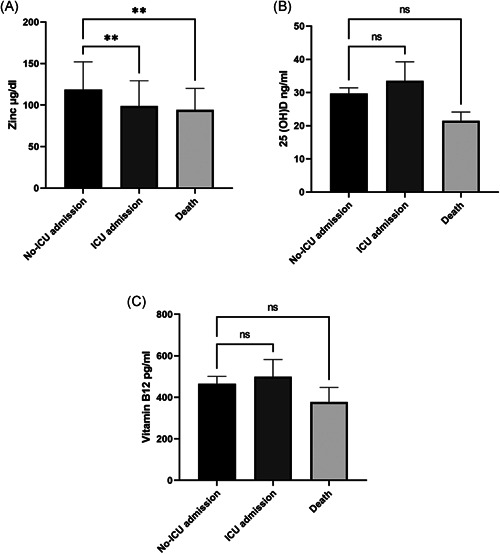
Comparison of serum concentrations of zinc (A), 25(OH)D (B), and vitamin B12 (C) at the time of admission between no‐ICU admission, ICU admission, and death groups in COVID‐19 patients. Data are the mean ± SEM. *p < 0.05 was considered statistically significant. ns = No significant, **p < 0.01. COVID‐19, coronavirus disease 2019; ICU, intensive care unit
It can be seen from the data in Table 2 that serum concentrations of zinc, vitamin B12, and 25(OH)D at the time of admission did not affect the length of hospital stay in COVID‐19 patients. Interestingly, we observed that serum 25 (OH)D levels in patients who did not need intubation were significantly higher than those who needed intubation (p = 0.006), while we did not find these significant differences in zinc and vitamin B12 (Table 2).
Table 2.
Comparison of length of hospital stay need for mechanical ventilation in COVID‐19 patients with serum concentrations of zinc, vitamin B12, and 25(OH)D
| Variable | Length of hospital stay | Intubation | ||||
|---|---|---|---|---|---|---|
| < 7 day | ≥ 7 days | p value | Need | No‐Need | p value | |
| Zinc | 114.9 ± 34.17 | 115.4 ± 34.17 | 0.92 | 122.5 ± 29.18 | 114.4 ± 33.92 | 0.37 |
| 25(OH)D | 26.38 ± 19.11 | 32.30 ± 19.64 | 0.13 | 15.01 ± 13.27 | 30.29 ± 19.42 | 0.006 |
| Vitamin B12 | 426.6 ± 45.13 | 532.5 ± 51.86 | 0.06 | 443.8 ± 68.08 | 490.9 ± 40.75 | 0.71 |
Note: Data are the mean ± SD. *p < 0.05 was considered statistically significant.
Abbreviations: COVID‐19, coronavirus disease 2019; SD, standard deviation.
4. DISCUSSION
In the current study, we registered demographic data and clinical characteristics of COVID‐19 patients and found the most common symptoms and frequency of comorbidities of COVID‐19 patients were similar to those in prior literature. 2 , 14
The European Food Safety Authority believes six vitamins (D, A, C, Folate, B6, and B12) and four minerals (zinc, iron, copper, and selenium) to be essential for the normal functioning of the immune system against infections. 15 Due to the known anti‐inflammatory and antiviral effects of zinc, 25(OH)D, and vitamin B12, in this study, we explored serum levels of these micronutrients in patients with COVID‐19 at the time of hospitalization. We also investigated the association between their serum levels and clinical outcomes.
In the present study, serum levels of zinc and 25(OH)D observed in COVID‐19 patients are higher than those observed by Im et al., 10 while the measured values for vitamin B12 are lower. These differences may be affected by the sample size, age, race, sex ratio, geographical location, and so forth. Consistent with this possibility, we showed that serum levels of vitamin D are closely related to sex and age. 16
Comparative analysis showed that serum zinc levels were significantly lower in patients who died than in those who were admitted to ICU or non‐ICU and survived (Figure 1). Following the present results, Jothimani et al. 13 showed decreased serum levels of zinc increase the mortality rate in COVID‐19 patients. However, their finding of the association between serum zinc levels and length of hospital stay was contrary to the present study. 13 In a meta‐analysis, Szarpak et al. 17 claimed that zinc supplementation does not have any beneficial impact on the clinical outcome of COVID‐19 patients. This differs from the findings presented here. 17
It seems possible that poor clinical outcome in COVID‐19 patients with decreased serum levels of zinc is due to this micronutrient decreasing replication of SARS‐CoV‐2 through inhibition of RNA polymerase activity, 18 modulating inflammatory responses, enhancing the cytotoxic activity of NK cells, and also increasing the phagocytic activity of macrophages. 7 , 19
It has previously been observed that vitamin D can protect against respiratory tract infections and reduce the risk of acute respiratory infections. 7 In the current study, we found that serum levels of 25(OH)D in the death group are lower than those of ICU and non‐ICU admission groups, although these differences were not statistically significant (Figure 1). This finding is consistent with that of Pizzini et al. 20 and showed that serum levels of vitamin D at onset of COVID‐19 are not associated with disease outcomes. However, this finding is contrary to previous studies, which have suggested that insufficiency or deficiency of 25(OH)D at the time of hospitalization increases mortality in COVID‐19 patients. 20 , 21 A recent meta‐analysis has shown that vitamin D levels or vitamin D supplementation only after the diagnosis of COVI‐19 but not before it can improve clinical outcome. 22
Regulation of antimicrobial proteins expression, inhibition of T cell proliferation, reduction of expression of pro‐inflammatory cytokines, and promotion of phagocytic ability of macrophages were proposed as the underlying mechanism of action vitamin D in COVID‐19 patients. 12 We also observed that serum 25 (OH)D levels in patients who did not the need for intubation significantly higher than those who the need for intubation. According to recent studies and our data, it seems possible that insufficiency or deficiency of vitamin D at the time of admission predispose COVID‐19 patients to acute respiratory distress syndrome following cytokine storms and eventually death.
Dos Santos, 23 in a review article, presented this hypothesis that vitamin B12 therapy can reduce severe damages of COVID‐19 through the improvement of circulation, reduction of oxidative stress, and action as an anti‐inflammatory and analgesic. Due to both the antiviral and anti‐inflammatory properties of vitamin B12, 6 we determined its serum levels in COVID‐19 patients at admission and found patients who died due to COVID‐19 had lower vitamin B12 compared with ICU and non‐ICU admission, but no significant difference among them (Figure 1). The results of this study partially support the findings from Tan et al. 11 that showed administration of vitamin B12 plus magnesium, and vitamin D in patients with COVID‐19 reduce the proportion of patients with clinical deterioration requiring oxygen support and, or intensive care support.
We found that zinc had a stronger association with the clinical outcome of COVID‐19 patients than vitamins D and B12. This result may be explained by previously published evidence that zinc is more efficient than vitamins D and B12 in boosting immune responses and antiviral activity against SARS‐CoV‐2 through inhibition of SARS‐coronavirus RNA polymerase activity, upregulation of antiviral proteins cleaving viral RNA, enhancement activities of NK and T cells, prevention of hyperimmune responses by modulating the cytokines and decreasing production of reactive nitrogen and oxygen species. 24 However, our result is contrary to that of Galmes et al. 15 who found vitamin D was more important than zinc and vitamin B12 for the treatment or prevention of COVID‐19. Due to genetic and environmental influences on nutrient intake, this inconsistency may be explained by the fact that genetic and/or environmental differences between study populations influences nutritional status. 25
This study had several limitations including: (1) It was conducted at a single center with a relatively small number of patients. (2) It is possible that patients recruited in a single center were more susceptible to severe COVID‐19 infection (e.g., older patients and comorbidities in patients). (3) It is possible that the different treatments selected for COVID‐19 patients can change clinical outcome. (4) It is possible that COVID‐19 infection influence indicators of nutritional status in the plasma through metabolic alterations.
5. CONCLUSION
In general, it seems that serum levels of 25(OH)D, vitamin B12, and especially zinc at the time of admission can influence clinical outcomes in COVID‐19 patients. Based on the results presented here, we suggest further studies (epidemiological studies, intervention studies, and clinical trial studies) are necessary to establish and characterize the benefits of vitamin D, vitamin B12, and especially zinc or even their combinations on COVID‐19 disease progression and clinical outcome.
CONFLICT OF INTERESTS
The authors declare that there are no conflicts of interest.
AUTHOR CONTRIBUTIONS
The author who conceived the study: Habibesadat Shakeri. Authors who designed the study: Habibesadat Shakeri and Hasan Namdar Ahmadabad. Authors who performed the experiments: Amir Azimian and Hamed Ghasemzadeh‐Moghaddam. Authors who performed statistical analyses: Mehdi Haresabadi and Tahereh Daneshmand. Authors who wrote and edited the manuscript: Mohammadreza Safdari and Hasan Namdar Ahmadabad
ACKNOWLEDGMENTS
This study was supported by the North Khorasan University of Medical Sciences, Bojnurd, Iran (grant no. 99/P/1372). The authors thank the members of the Department of Pathobiology and Medical Laboratory Sciences, North Khorasan University of Medical Sciences, Bojnurd, Iran for their technical assistance.
Shakeri H, Azimian A, Ghasemzadeh‐Moghaddam H, et al. Evaluation of the relationship between serum levels of zinc, vitamin B12, vitamin D, and clinical outcomes in patients with COVID‐19. J Med Virol. 2021;94:141‐146. 10.1002/jmv.27277
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Singhal T. A review of coronavirus disease‐2019 (COVID‐19). Indian J Pediatrics. 2020;87(4):281‐286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sheikhi K, Shirzadfar H, Sheikhi M. A review on novel coronavirus (COVID‐19): symptoms, transmission and diagnosis tests. Res Infect Dis Trop Med. 2020;2(1):1‐8. [Google Scholar]
- 3. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Abdin SM, Elgendy SM, Alyammahi SK, Alhamad DW, Omar HA. Tackling the cytokine storm in COVID‐19, challenges, and hopes. Life Sci. 2020;257:118054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hashemi S‐A, Safamanesh S, Ghasemzadeh‐Moghaddam H, et al. Report of death in children with SARS‐CoV‐2 and human metapneumovirus (hMPV) coinfection: is hMPV the trigger? J Med Virol. 2020;93:579‐581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gombart AF, Pierre A, Maggini S. A review of micronutrients and the immune system–working in harmony to reduce the risk of infection. Nutrients. 2020;12(1):236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maggini S, Pierre A, Calder PC. Immune function and micronutrient requirements change over the life course. Nutrients. 2018;10(10):1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Beveridge S, Wintergerst E, Maggini S, Hornig D. Immune‐enhancing role of vitamin C and zinc and effect on clinical conditions. Ann Nutr Metab. 2006;50(2):85–94. https://www.karger.com/Article/Abstract/90495 [DOI] [PubMed] [Google Scholar]
- 9. Herrmann W, Obeid R. Causes and early diagnosis of vitamin B12 deficiency. Dtsch Arztebl Int. 2008;105(40):680‐685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Im JH, Je YS, Baek J, Chung M‐H, Kwon HY, Lee J‐S. Nutritional status of patients with COVID‐19. Int J Infect Dis. 2020;100:390‐393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tan CW, Ho LP, Kalimuddin S, et al. Cohort study to evaluate effect of vitamin D, magnesium, and vitamin B12 in combination on severe outcome progression in older patients with coronavirus (COVID‐19). Nutrition. 2020;79:111017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ahmed F. A network‐based analysis reveals the mechanism underlying vitamin D in suppressing cytokine storm and virus in SARS‐CoV‐2 infection. Front Immunol. 2020;11:3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jothimani D, Kailasam E, Danielraj S, et al. COVID‐19: poor outcomes in patients with zinc deficiency. Int J Infect Dis. 2020;100:343‐349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huang X, Wei F, Hu L, Wen L, Chen K. Epidemiology and clinical characteristics of COVID‐19. Arch Iran Med. 2020;23(4):268‐271. [DOI] [PubMed] [Google Scholar]
- 15. Galmés S, Serra F, Palou A. Current state of evidence: influence of nutritional and nutrigenetic factors on immunity in the COVID‐19 pandemic framework. Nutrients. 2020;12(9):2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shakeri H, Pournaghi S‐J, Hashemi J, Mohammad‐Zadeh M, Akaberi A. Do sufficient vitamin D levels at the end of summer in children and adolescents provide an assurance of vitamin D sufficiency at the end of winter? A cohort study. J Pediatr Endocrinol Metab. 2017;30(10):1041‐1046. [DOI] [PubMed] [Google Scholar]
- 17. Szarpak L, Pruc M, Gasecka A, et al. Should we supplement zinc in COVID‐19 patients? Evidence from meta‐analysis. Pol Arch Med Wewn. Published online June 28, 2021. [DOI] [PubMed]
- 18. Te Velthuis AJ, van den Worm SH, Sims AC, Baric RS, Snijder EJ, van Hemert MJ. Zn2+ inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6(11):e1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pal A, Squitti R, Picozza M, et al. Zinc and COVID‐19: basis of current clinical trials. Biol Trace Elem Res. 2020;199:1‐11. https://link.springer.com/article/10.1007%2Fs12011-020-02437-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pizzini A, Aichner M, Sahanic S, et al. Impact of vitamin D deficiency on COVID‐19—a prospective analysis from the CovILD Registry. Nutrients. 2020;12(9):2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pereira M, Dantas Damascena A, Galvão Azevedo LM, Almeida Oliveira T, Santana JM. Vitamin D deficiency aggravates COVID‐19: systematic review and meta‐analysis. Crit Rev Food Sci Nutr. 2020;4:1‐9. [DOI] [PubMed] [Google Scholar]
- 22. Pal R, Banerjee M, Bhadada S, Shetty A, Singh B, Vyas A. Vitamin D supplementation and clinical outcomes in COVID‐19: a systematic review and meta‐analysis. J Endocrinol Invest. 2021:1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lopes Monyck Jeane dos Santos . Can vitamin B12 be an adjuvant to COVID‐19 treatment?. GSC Biological and Pharmaceutical Sciences. 2020;11:(3):001‐005. 10.30574/gscbps.2020.11.3.0155 [DOI] [Google Scholar]
- 24. Samad N, Sodunke TE, Abubakar AR, et al. The implications of zinc therapy in combating the COVID‐19 global pandemic. J Inflamm Res. 2021;14:527‐550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu J, Tuvblad C, Raine A, Baker L. Genetic and environmental influences on nutrient intake. Genes Nutr. 2013;8(2):241‐252. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


