Abstract
Introduction
Coronavirus disease‐2019 (COVID‐19) is a respiratory disease whose clinical manifestation ranges from asymptomatic to severe respiratory failure. The purpose of this study was to investigate the place of serum surfactant‐D (SP‐D) and angiopoetin‐2 (Ang‐2) levels in predicting severity of disease in patients diagnosed with COVID‐19.
Methods
Sixty‐four patients diagnosed with COVID‐19 between September 2020 and February 2021, 50 patients diagnosed with community‐acquired pneumonia and a 50‐member healthy control group were included in the study. Plasma samples and clinical data were collected within 72 h after admission, during hospital stay. Serum SP‐D and Ang‐2 concentrations were measured using the enzyme‐linked immunosorbent assay.
Results
SP‐D and Ang‐2 levels were significantly higher in the mild–moderate pneumonia and severe/critical patient groups compared to the asymptomatic and noncomplicated COVID‐19 patients (p < 0.001 for all groups). Serum SP‐D and Ang‐2 levels of severe‐critical COVID‐19 patients were significantly higher than CAP patients (p < 0.001). Powerful correlation was present between clinical severity of COVID‐19 and SP‐D and Ang‐2 levels (r = 0.885 p < 0.001 and r = 0.913 p < 0.001, respectively). Cut‐off values of 37.7 ng/ml (AUC = 0.763, p < 0.001, 95% confidence interval [CI] = 0.667–0.860) for SP‐D and 4208.3 pg/ml (AUC = 0.659, p = 0.004, 95% CI = 0.554–0.763) for Ang‐2 were identified as predictors of COVID‐19 disease at receiver operating characteristic curve analysis.
Conclusion
SP‐D and Ang‐2 are predictive factors in differentiating COVID‐19 patients and determining severity of disease. These data may be important for the initiation of treatment in the early stage of the disease in patients with COVID‐19.
Keywords: angiopoetin‐2, COVID‐19, lung injury, serum surfactant‐D
Highlights
-
‐
All clinicians should be aware of cutaneous lesions of COVID‐19
-
‐
Such manifestations could be the first presentation of the infection or an indicator of the deterioration of the patient s wellbeing.
-
‐
Also, such dermatologic symptoms could be because of anti‐SARS‐CoV‐2 medications, expressing the need to monitor patients cautiously.
1. INTRODUCTION
A novel coronavirus was identified as the agent in a series of cases of pneumonia in the Chinese city of Wuhan in the province of Hubei in late 2019. The virus responsible for the outbreak in China subsequently spread rapidly across the entire world. The virus responsible for 2019 Coronavirus disease (COVID‐19) was given the name Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2).1 Infected patients may be asymptomatic, or else the condition may manifest from a mild clinic form to severe respiratory failure accompanied by symptoms such as sore throat, fatigue, joint pain, and loss of smell and taste.2
Surfactant‐D (SP‐D) is released from Type II alveolar cells. In case of infection it is converted into trimeric form as a result of various chemical modifications and proteolytic breakdown in the lung, and production increases. Pulmonary SP‐D enters the circulation as a result of loss of integrity of the air–blood barrier in the lungs. Circulatory SP‐D is a biomarker showing lung damage.3 Serum SP‐D levels have been shown to be associated with the development, progression, and severity of various pulmonary diseases.4 SARS‐Cov‐2 impairs pulmonary surfactant production by causing the breakdown of type II pneumocytes in the lung, and results in dyspnea, acute respiratory distress syndrome (ARDS), and respiratory failure in Covid‐19 patients.5
Endothelial dysfunction plays a central role in the pathophysiology of COVID‐19. Postmortem studies have shown widespread inflammatory infiltration and endothelial damage in the pulmonary and extrapulmonary capillary bed.6 Endothelial damage is a common cause of thrombosis, a cause of morbidity and mortality in COVID‐19 patients, ARDS, and multiple organ failure.7 Angiopoietin‐2 (Ang‐2) increases endothelial inflammation and permeability by acting as an Ang‐1 and Tie2 signaling antagonist.8 Higher serum Ang‐2 levels have been reported as a result of induction of endothelial permeability occurring in pneumonia and ARDS compared to healthy individuals, and Ang‐2 levels have been shown to be correlated with the severity of sepsis and pneumonia.9, 10
Several parameters are employed as prognostic factors in COVID‐19.11 However, these parameters are not specific in COVID‐19 infection. The purpose of the present study was therefore to evaluate serum SP‐D and Ang‐2 levels, showing endothelial dysfunction and respiratory failure, for predicting disease severity in COVID‐19 patients at time of presentation and for differentiating these from patients with community‐acquired pneumonia (CAP).
2. MATERIALS AND METHODS
2.1. Study design and participants
Sixty‐four adult COVID‐19 patients treated and followed‐up at the Atatürk University Medical Faculty Hospital, Turkey, between September 2020 and February 2021, and 50 CAP patients were included in the study. Fifty asymptomatic volunteers were enrolled as an adult control group. Approval for the study was granted by the Atatürk University Medical Faculty Clinical Research Ethical Committee (no. B.30.2. ATA.0.01.00/201).
2.2. Definitions and diagnosis
Group 1 consisted of patients aged over 18 tested for SARS‐CoV‐2 infections using nasopharyngeal swabs, reported as reverse‐transcription polymerase chain reaction (RT‐PCR) positive, and diagnosed with COVID‐19 based on the Turkish Ministry of Health COVID‐19 Adult Patient Treatment Guideline and literature.12, 13, 14 These were divided into four subgroups based on clinical characteristics—asymptomatic, uncomplicated, mild–moderate pneumonia, and severe pneumonia/critical. Clinical evaluation of all patients was performed using thoracic computed tomography (CT) scanning and laboratory assessments.
Asymptomatic patients can develop symptoms in a median 4 days (3–7 days) after a positive RT‐PCR test.15 Patients with no symptoms 4 days after first RT‐PCR positivity were therefore regarded as asymptomatic in this study.
Uncomplicated patients consisted of individuals with findings of fever, muscle/joint pains, cough, sore throat, and nasal congestion, without respiratory distress, tachypnea, or SPO2 < 93%, with no poor prognostic criteria at blood tests (blood lymphocyte count 800 cells/µl or ferritin >500 ng/ml or d‐dimer >1000 ng/ml, etc.), and with normal lung radiographs.
Patients with mild–moderate pneumonia were defined as individuals with findings such as fever, muscle/joint pain, cough, and sore throat, with a respiratory rate <30/min, SpO2 levels in room air >90%, and mild–moderate pneumonia findings on lung X‐rays or tomography.
Patients with severe pneumonia had findings such as fever, muscle/joint pain, cough, and sore throat, with tachypnea (≥30/min), SpO2 levels in room air ≤90%, and findings of diffuse bilateral pneumonia on X‐rays or tomography.
Group 2 consisted of patients with fever (>38.3°), cough, and phlegm, with chest X‐rays compatible with infiltration (lobar consolidation) and leukocytosis, diagnosed with community‐acquired bacterial pneumonia,16 and with CAP but identified as SARS‐CoV‐2 infection‐negative with nasopharyngeal swabs. These had no history of COVID‐19 patients.
Group 3 was made up of healthy, asymptomatic adults with normal physical examination and routine test findings. These were SARS‐CoV‐2 RT‐PCR‐negative at the time of inclusion in the study.
2.3. Analyte assay techniques
SP‐D and Ang‐2 levels were measured with the ELISA method using a Human SP‐D ELISA Kit (Elabscience, HUMAN SP‐D: CAT LOG NO:E‐EL‐H1269) and a Human ANG2 ELISA Kit (Elabscience, HUMAN ANG:E‐EL‐H0008) in line with the manufacturer's instructions. Kit measurement ranges for SP‐D and Ang‐2 were 0–100 ng/ml and 0–3000 pg/ml, respectively.
2.4. Statistical analysis
SPSS 20.0 for Windows software (SPSS Inc.) was employed for data recording and statistical analysis. Descriptive statistics were expressed as number and percentage for categorical variables and as mean ± SD for numerical variables. Normality of distribution was assessed using the Kolmogorov Smirnov test. One‐way analysis of variance (ANOVA) was used to compare study groups, and the degree of significance between groups was determined using the post hoc Tukey test. Statistical analysis of the difference between the groups in terms of gender distribution was performed with the χ 2 test. Relationships between results were evaluated using Pearson analysis. The receiver operating characteristic (ROC) curve, an expression of a specific method's predictive power, was used to calculate SP‐D and Ang‐2 sensitivity, specificity, area under the curve (AUC) and cut‐off values. A value of p < 0.05 were regarded as statistically significant.
3. RESULTS
Thirty‐five (54.7%) of the patients included in Group 1 were women, and 29 (45.3%) were men, while 22 (44%) patients in Group 2 were women and 28 (56%) were men. Twenty‐nine (58%) members of the control group were women and 21 (42%) were men. Mean ages were 55.17 ± 16.1 years in Group 1, 51.3 ± 14.5 in Group 2, and 58.6 ± 15.7 in the control group. There was no difference between the groups in terms of gender or age (p = 0.66 and p = 0.337, respectively). When the COVID‐19 patients were classified according to their clinical characteristics, 11 (17.2%) were asymptomatic, 13 (20.3%) were uncomplicated, 22 (34.4%) had mild–moderate pneumonia, and 18 (28.1%) were severe/critical. The groups' clinic and demographic characteristics are shown in Table 1. The groups' routine laboratory parameters are shown in Table 2.
Table 1.
Demographics, clinical characteristics of the groups
| Group 1 (n = 64) | Group 2 (n = 50) | Group 3 (n = 50) | |
|---|---|---|---|
| Gender n(%) | |||
| Female | 35 (54.7%) | 22 (44%) | 29 (58%) |
| Male | 29 (45.3%) | 28 (56%) | 21 (42%) |
| Mean ages (years) | 55.17 ± 16.1 | 51.3 ± 14.5 | |
| COVID‐19 clinical classification | |||
| Asymptomatic | 11 (17.2%) | – | – |
| Uncomplicated | 13 (20.3%) | – | – |
| Mild–moderate pneumonia | 22 (34.4%) | – | – |
| Severe/critical | 18 (28.1%) | – | – |
| Any comorbidities | 16 (25%) | 11 (22%) | – |
| Hypertension | 5 (8%) | 3 (6%) | – |
| Diabetes mellitus | 7 (11%) | 5(10%) | – |
| Chronic renal failure | 2 (3%) | 1 (2%) | – |
| COPD | 2 (3%) | 2 (4%) | – |
| Presentation | |||
| Fever | 21 (32.8%) | 35 (70%) | – |
| Cough | 18 (28%) | 28 (56%) | – |
| Fatigue | 21 (32.8%) | 45 (90%) | – |
| Myalgia | 16 (25%) | 38 (76%) | – |
| Sputum production | – | 25 (5%) | – |
| Short of breath | 11 (17%) | – | – |
| Throat ache | 10 (15.6%) | – | – |
| Headache | 9 (14%) | 28 (56%) | – |
| Nausea or vomiting | 7 (10.9%) | – | – |
| Loss of taste‐smell | 6 (9.4%) | – | – |
| Diarrhea | 4 (6%) | – | – |
| Back pain | 4 (6%) | 18 (36%) | – |
| Chills | 1 (1.5%) | ||
| Intensive care requirement | 6 (9.4%) | – | – |
| Length of hospitalization (days) | |||
| Median (min–max) | 8 (2‐30) | 7(5‐22) | – |
| Mortality | 4 (6.3%) | – | – |
Abbreviation: COPD, chronic obtstructive pulmonary disease.
Table 2.
Group 1, Group 2, and control group laboratory parameters
| Group 1 (n = 64) | Group 2 (n = 50) | Control (n = 50) | p value | |
|---|---|---|---|---|
| WBC (cells/µl) | 5458.1 ± 1992.4 | 14782.6 ± 2678.8 | 7357.9 ± 1859.7 | <0.001a, b, c |
| Lymphocytes (cells/µl) | 1315.1 ± 654.3 | 1603.9 ± 448.9 | 2704.2 ± 1529.1 | 0.258a |
| < 0.001b, c | ||||
| Neutrophils (cells/µl) | 3301.6 ± 1818.9 | 12988.0 ± 2363.5 | 4135.6 ± 1409 | <0.001a, c |
| 0.055b | ||||
| LDH (U/L) | 324.5 ± 139.7 | 231.7 ± 73.4 | 197.4 ± 37.6 | < 0.001a, b |
| 0.193c | ||||
| Procalcitonin (ng/ml) | 1.04 ± 5.37 | 0.22 ± 0,29203 | 0.09 ± 0.12 | 0.397a |
| 0.293b | ||||
| 0.980c | ||||
| CRP (mg/L) | 71.7 ± 131.7 | 81.7 ± 34.9 | 2.08 ± 0.74 | 0.804a |
| < 0.001b, c | ||||
| ESR (mm/h) | 24.2 ± 21.6 | 39 ± 14.9 | 8.9 ± 3.6 | < 0.001a, b, c |
| Ferritin (ng/ml) | 598.3 | 235.9 ± 126.8 | 212.9 ± 60.8 | 0.005a |
| 0.003b | ||||
| 0.980c | ||||
| d‐Dimer (ng/ml) | 1284.8 ± 1070.5 | 315 ± 134.01 | 191.7 ± 52.4 | < 0.001a, b |
| 0.632c |
Abbreviations: CRP, C‐reactive protein; ESR, erythrocyte sedimentation rate; LDH, lactate dehydrogenase; WBC, white blood cells.
Between Group 1 and Group 2.
Between Group 1 and the control group.
Between Group 2 and the control group.
SP‐D and Ang‐2 levels were compared among the patients in Group 1 according to their clinical severity. SP‐D and Ang‐2 levels were significantly higher in the mild–moderate pneumonia and severe/critical patient groups compared to the asymptomatic and uncomplicated COVID‐19 patients (p < 0.001 for all groups. In addition, severe/critical COVID‐19 patients have higher serum SP‐D and Ang‐2 than CAP patients. (p < 0.001) (Table 3).
Table 3.
A comparison of Group 1 and 2 patients' SP‐D and Ang‐2 levels
| Group 1 | Group 2 | |||||
|---|---|---|---|---|---|---|
| Asymptomatic n: 11 | Uncomplicated patients n: 13 | Mild‐moderate pneumonia n: 22 | Severe/critical n: 18 | n: 50 | p value | |
| SP‐D (ng/ml) | 4.5 ± 1.8 | 4.4 ± 1.7c | 28.9 ± 7.8 | 60 ± 12.9 | 47.3 ± 7.5 | <0.001a, b |
| Ang‐2 (pg/ml) | 720.5 ± 279.1 | 1061.1 ± 273.3c | 3951.1 ± 824.7 | 7089.3 ± 1263.4 | 4883.4 ± 2146.3 | <0.001a, b |
Note: Values expressed as mean ± SD.
Abbreviations: Ang‐2, angiopoetin‐2; SP‐D, surfactant protein D.
Two‐way comparisons for all groups (apart from the asymptomatic and noncomplicated groups).
The comparison of Group 2 and Group 1 subgroups.
p > 0.05, Comparison of the asymptomatic and uncomplicated groups.
Significant, powerful positive correlation was also determined between serum SP‐D and Ang‐2 levels and between SP‐D/Ang‐2 with the clinical severity of COVID‐19 (r = 0.910, p < 0.01) (Figure 1). Positive significant correlation was determined between COVID‐19 patients' clinical severity and serum SP‐D, Ang‐2, d‐dimer, ESR, CRP, ferritin, and LDH levels, while clinical severity was nonsignificantly correlated with PCT (Table 4). The most powerful correlation between these parameters and COVID‐19 patient clinical severity was exhibited by d‐dimer then SP‐D and Ang‐2 levels, respectively (r = 0.885 p < 0.001 and r = 0.913 p < 0.001, respectively).
Figure 1.
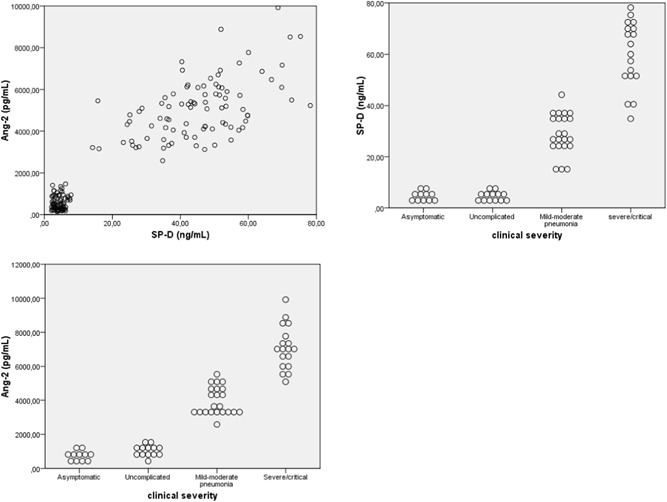
The dot plots graphic the correlation between SP‐D and Ang‐2 and between SP‐D/Ang‐2 with the clinical severity of disease, Ang‐2, angiopoetin‐2; SP‐D, surfactant protein D
Table 4.
Correlations between COVID‐19 patients' clinical severity and SP‐D, Ang‐2, ESR, CRP, ferritin, d‐dimer, PCT, and LDH parameters
| SP‐D | Ang‐2 | LY | ESR | CRP | Ferritin | d‐dimer | PCT | LDH | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Clinical severity | Correlation coefficient (r) | 0.885 | 0.913 | −0.635 | 0.560 | 0.515 | 0.585 | 0.946 | 0.228 | 0.393 |
| p value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.7 | 0.01 |
Abbreviations: Ang‐2, angiopoetin‐2; CRP, C‐reactive protein; ESR, erythrocyte sedimentation rate; LDH, lactate dehydrogenase; PCT, procalcitonin; SP‐D, surfactant protein D.
SP‐D and Ang‐2 levels were significantly and positively correlated with WBC, d‐dimer, ESR, ferritin, CRP, PCT, and LDH, and significantly and negatively correlated with LY (p = 0.007 for SP‐D and PCT, and p = 0.032 for Ang‐2 and PCT, p < 0.001 for all other parameters) (Table 5).
Table 5.
Correlations between COVID‐19 patients' SP‐D and Ang‐2 levels and WBC, LY, d‐dimer, ESR, ferritin, CRP, PCT, and LDH parameters
| WBC | LY | d‐dimer | ESR | Ferritin | CRP | PCT | LDH | ||
|---|---|---|---|---|---|---|---|---|---|
| SP‐D | Correlation coefficient (r) | 0.403 | −0.552 | 0.513 | 0.692 | 0.414 | 0.534 | 0.209 | 0.319 |
| p value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.007 | <0.001 | |
| Ang‐2 | Correlation coefficient (r) | 0.290 | −0.619 | 0.522 | 0.638 | 0.432 | 0.588 | 0.168 | 0.416 |
| p value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.032 | <0.001 |
Abbreviations: Ang‐2, angiopoetin‐2; CRP, C‐reactive protein; ESR, erythrocyte sedimentation rate; LDH, lactate dehydrogenase; LY, lymphocyte; PCT, procalcitonin; SP‐D, surfactant protein D; WBC, white blood cell.
ROC curve analysis was applied to determine the diagnostic sensitivity and specificity of serum SP‐D and Ang‐2 levels in patients diagnosed with COVID‐19. At a cut‐off value of 37.7, serum SP‐D levels exhibited sensitivity of 90% and specificity of 72% in differentiating COVID‐19 cases from healthy individuals (AUC = 0.763, p < 0.001, 95% confidence interval [CI] = 0.667–0.860). At a cut‐off value of 4208.3, serum Ang‐2 levels exhibited 75% sensitivity and 63% specificity in differentiating COVID‐19 cases from healthy individuals (AUC = 0.659, p = 0.004, 95% CI = 0.554–0.763) (Figure 2A).
Figure 2.
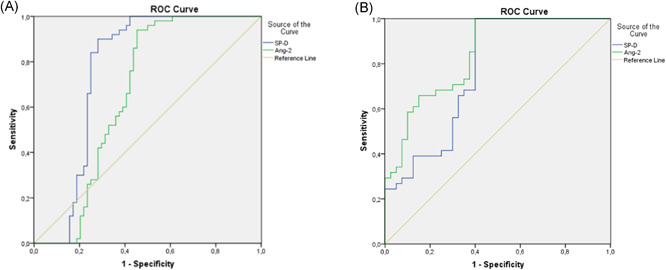
(A) ROC curve analysis showing the diagnostic sensitivity and specificity of serum SP‐D and Ang‐2 levels in patients with COVID‐19. (B) ROC curve analysis showing the diagnostic sensitivity and specificity of serum SP‐D and Ang‐2 levels for endothelial dysfunction. Ang‐2, angiopoetin‐2; ROC, receiver‐operating characteristic curve; SP‐D, surfactant protein D
ROC curve analysis was applied to determine the diagnostic sensitivity and specificity of serum SP‐D and Ang‐2 levels in patients diagnosed with COVID‐19. At a cut‐off value of 43.4, serum SP‐D levels exhibited sensitivity of 78% and specificity of 69% in differentiating COVID‐19 endothelial dysfunction (AUC = 0.798, p < 0.001, 95% CI = 0.662‐0.874). At a cut‐off value of 5207.3 pg/ml, serum Ang‐2 levels exhibited 82% sensitivity and 73% specificity in differentiating COVID‐19 endothelial dysfunction (AUC = 0.882, p < 0.001, 95% CI = 0.757–0.925) (Figure 2B).
4. DISCUSSION
Serum SP‐D and Ang‐2 levels were higher in the patients with COVID‐19 in this study than in those the healthy control group. High serum SP‐D and Ang‐2 levels in severe‐critical COVID‐19 patients are significant in differentiating them from CAP patients. SP‐D and Ang‐2 elevation was associated with severe disease in COVID‐19. Positive and significant correlation was determined between WBC, ESR, d‐dimer, ferritin, CRP, and PCT levels frequently employed in the follow‐up of COVID‐19 and serum SP‐D and Ang‐2 levels, and significant and negative correlation was observed between serum SP‐D and Ang‐2 levels and LY.
SP‐D is released by type II alveolar cells, and increased serum concentrations are a result of protein translocation emerging with impairment of the structural integrity of the alveolar‐capillary membrane.17 Various clinical studies have recommended the use of serum SP‐D levels as a biomarker in acute and chronic respiratory diseases, such as chronic obstructive pulmonary disease (COPD) and idiopathic pulmonary fibrosis.18, 19 SP‐D plays a central role in pulmonary host defense and in the regulation of the inflammatory response, and serum levels in critical patients change as a result of pulmonary parenchyma damage. Evidence from wide cohorts emphasizes that it is a prognostic marker of clinical flare‐ups and COPD progression.19 In addition, it has been shown to be useful in predicting the risk of poorer outcomes in critical patients A/H1N1 virus infection.20 SARS‐Cov‐2 causes alveolar damage by infecting alveolar type II cells involved in pulmonary surfactant production. Asymptomatic patients without pulmonary parenchymal involvement and noncomplicated patients with upper respiratory tract symptoms only can be followed‐up at home with symptomatic therapies, although patients with lung infiltrations and progressing to ARDS experience severe disease.
Endothelial dysfunction and/or impaired angiogenesis causes microvascular dysfunction in all organs. Endothelial dysfunction and angiogenesis have been identified as a marker of disease severity in several conditions.9, 10 Ang‐2 stored in Weibel Palade bodies regulates angiogenesis. Ang‐2 is released as a result of endothelial activation by inflammatory cytokines or thrombin and induces inflammation and vascular hyperpermeability.21 An antithrombotic environment develops as a result of IL‐6‐related inflammation in COVID‐19 patients, and the endothelial barrier is compromised, leading to coagulopathy, various complications, and mortality.22, 23 Novel biomarkers with high specificity are therefore needed to determine disease severity in the early stage and to prevent morbidity and mortality in COVID‐19 patients.
CAPs are some of the most widespread infectious diseases requiring hospitalization and leading to mortality worldwide. Although several bacterial pathogens are involved in the etiology, the most frequent agent is Streptococcus pneumoniae. Viral pathogens are also implicated in the etiology.16 Differentiating CAP from COVID‐19 patients during the pandemic and the taking of early isolation measures is important in terms of the early initiation of protective therapies. The clinical appearance of lung damage associated with SARS‐Cov infection was described as similar to that of CAP in previous years.24 Practical and highly specific biomarker parameters are therefore needed to assist with differentiating COVID‐19 patients from CAP.
Several hematological inflammatory markers and laboratory parameters associated with the severity of COVID‐19 have therefore been evaluated. One such parameter, WBC, changes in COVID‐19 patients. Lymphopenia is most frequently observed, although leukocytosis or leukopenia have also been reported.25 Lymphopenia is a maker used in the hospitalization of COVID‐19 patients and in determining disease severity.26 One meta‐analysis reported that severe COVID‐19 may be associated with a high WBC count and a lower lymphocyte count.27 WBC values were lower in COVID‐19 patients than in the CAP and control groups in the present study, while WBC levels in patients with CAP were higher than in the COVID‐19 and control groups.
In meta‐analysis studies, high ESR, CRP, PCT, and ferritin levels have been reported in severe COVID‐19.11, 27 LDH, d‐dimer, ESR, CRP, ferritin, and PCT levels associated with clinical course and prognosis inpatients diagnosed with COVID‐19 were higher than those in the CAP and control groups in the present study, while LY values were lower than in the CAP and control groups. Additionally, positive significant correlation was determined between disease severity and ESR, CRP, ferritin, d‐dimer, and LDH, and significant negative correlation with LY.
Severe acute respiratory syndrome coronavirus (SARS‐Cov) resulted in significant morbidity and mortality in humans in previous years. Studies have reported that no significant difference between patients with SARS and those with bacterial pneumonia in terms of pulmonary infiltration, chest X‐ray scores, thrombocytopenia and leukocytopenia. However, higher serum SP‐D levels have been shown compared to CAP patients and healthy controls.26 One study evaluating serum SP‐D levels in patients with CAP reported below‐normal values at the time of patients' presentation to hospital. Initially low SP‐D levels were then observed to peak on the fifth day of follow‐up. That study also reported no correlation between SP‐D and CRP concentrations, and that these molecules respond independently to bacterial infection.28
Higher serum SP‐D levels have been reported in COVID‐19 patients compared to a control group, and in patients developing ARDS and macrophage activation syndrome. The increase in SP‐D levels was described as associated with the clinical severity of the disease.29 In the present study, severe/critical COVID‐19 patients have higher serum SP‐D and Ang‐2 than CAP patients. In addition, we also determined in increase in serum SP‐D level in association with the severity of the disease. Serum SP‐D levels in patients diagnosed with CAP and COVID‐19 are more useful in differentiating COVID‐19 patients and determining disease severity than other routinely used parameters. They also exhibit high specificity in differentiating COVID‐19 patients from healthy individuals.
SARS‐CoV‐2 binds to ACE‐2 receptors expressed in endothelial cells and causes endothelial damage. An increase in procoagulant factors and coagulopathy also occurs through the effect of inflammatory cytokines during COVID‐19 infection. This in turn leads to high d‐dimer levels, shown to be linked to mortality.23 High Ang‐2 levels have been observed in COVID‐19 patients admitted to intensive care, and these have been reported to be a good marker showing endothelial dysfunction in patients with COVID‐19.30 Ang‐2 levels have been shown to increase in patients with ARDS and sepsis progressing with widespread endothelial damage and to be predictive of mortality in patients with ARDS.31 Ang‐2 levels in the present study were significantly higher in patients diagnosed with COVID‐19 than the healthy controls. In addition, Ang‐2 levels of severe‐critical COVID‐19 patients were higher than CAP patients. Positive correlation was present between d‐dimer and Ang‐2, showing endothelial dysfunction. Powerful correlation was observed between severity of disease in COVID‐19 patients and Ang‐2 levels. Since an increased degree of pulmonary endothelial damage may be a coindicator of the disease severity, serum Ang‐2 levels are also an excellent guiding parameter in determining severity in COVID‐19 patients.
In conclusion, our findings support the idea of a powerful association between SP‐D and Ang‐2 levels in the diagnosis of COVID‐19 patients and in the severity of the disease. SP‐D levels indicating alveolar damage rise in COVID‐19 patients. Ang‐2 levels indicating endothelial dysfunction in COVID‐19 patients may be a guide to the initiation of protective vascular therapies in the early stage of the disease to prevent thrombotic complications. The current findings support the idea of the use of serum SP‐D and Ang‐2 in the early identification of pulmonary and endothelial damage in COVID‐19 and the use of preventive treatments in the early period.
AUTHOR CONTRIBUTIONS
Handan Alay: Concept, design, supervision, resources, materials, data collection and/or processing, literature search, writing manuscript. Esra Laloglu: Concept, design, supervision, analysis and/or interpretation, literature search, writing manuscript.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
Alay H, Laloglu E.. The role of angiopoietin‐2 and surfactant protein‐D levels in SARS‐CoV‐2‐related lung injury: A prospective, observational, cohort study. J Med Virol. 2021;93:6008‐6015. 10.1002/jmv.27184
Contributor Information
Handan Alay, Email: alayhandan@gmail.com.
Esra Laloglu, Email: dresralaloglu@hotmail.com.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.World Health Organization . Director‐General's remarks at the media briefing on 2019‐nCoV on 11 February 2020. http://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020. Accessed March 12, 2021.
- 2.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sorensen GL. Surfactant protein D in respiratory and non‐respiratory diseases. Front Med. 2018;5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ware LB, Koyama T, Zhao Z, et al. Biomarkers of lung epithelial injury and inflammation distinguish severe sepsis patients with acute respiratory distress syndrome. Crit Care. 2013;17:R253. 10.1186/cc13080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghati A, Dam P, Tasdemir D, et al. Exogenous pulmonary surfactant: a review focused on adjunctive therapy for severe acute respiratory syndrome coronavirus 2 including SA‐A and SP‐D as added clinical marker. Curr Opin Colloid Interface Sci. 2021;51:101413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bradley BT, Maioli H, Johnston R, et al. Histopathology and ultrastructural findings of fatal COVID‐19 infections in Washington State: a case series. The Lancet. 2020;396:320‐332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pons S, Fodil S, Azoulay E, Zafrani L. The vascular endothelium: the cornerstone of organ dysfunction in severe SARS‐CoV‐2 infection. Crit Care. 2020;24:353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thurston G, Daly C. The complex role of angiopoietin‐2 in the angiopoietin‐tie signaling pathway. Cold Spring Harb Perspect Med. 2012;2:a006550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gutbier B, Neuhauß AK, Reppe K, et al. Prognostic and pathogenic role of angiopoietin‐1 and ‐2 in pneumonia. Am J Respir Crit Care Med. 2018;198:220‐231. [DOI] [PubMed] [Google Scholar]
- 10.Lymperopoulou K, Velissaris D, Kotsaki A, et al. Angiopoietin‐2 associations with the underlying infection and sepsis severity. Cytokine. 2015;73:163‐168. [DOI] [PubMed] [Google Scholar]
- 11.Elshazli RM, Toraih EA, Elgaml A, et al. Diagnostic and prognostic value of hematological and immunological markers in COVID‐19 infection: a meta‐analysis of 6320 patients. PLOS One. 2020;15(8):e0238160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shi Y, Wang G, Cai XP, et al. An overview of COVID‐19. J Zhejiang Univ‐Sci B (Biomed & Biotechnol). 2020;21(5):343‐360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Republic of Turkey Ministry of Health , General Directorate of Public Health. COVID‐19 (SARS‐CoV‐2 INFECTION). Adult Patient Treatment. https://covid19bilgi.saglik.gov.tr/depo/rehberler/covid-19-rehberi/COVID-19_REHBERI_ERISKIN_HASTA_TEDAVISI.pdf
- 14.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (covid‐19) outbreak in China: summary of a report of 72 314 cases from the chinese center for disease control and prevention. JAMA. 2020;323:1239‐1242. [DOI] [PubMed] [Google Scholar]
- 15.Sakurai A, Sasaki T, Kato S, et al. Natural history of asymptomatic SARS‐CoV‐2 infection. N Engl J Med. 2020;383:885‐886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community‐acquired pneumonia. an official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang L, Ikegami M, Korfhagen T. Neither SP‐A nor NH2‐terminal domains of SP‐A can substitute for SP‐D in regulation of alveolar homeostasis. Am J Physiol Lung Cell Mol Physiol. 2006;291:181‐190. [DOI] [PubMed] [Google Scholar]
- 18.Greene KE, KingTE, Jr., Kuroki, Y, et al. Serum surfactant proteins‐A and ‐D as biomarkers in idiopathic pulmonary fibrosis. Eur Respir J. 2002;19:439‐446. [DOI] [PubMed] [Google Scholar]
- 19.Sin DD, Leung R, Gan WQ, Man SP. Circulating surfactant protein D as a potential lung‐specific biomarker of health outcomes in COPD: a pilot study. BMC Pulm Med. 2007;7:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delgado C, Krötzsch E, Jiménez‐Alvarez LA, et al. Serum surfactant protein D (SP‐D) is a prognostic marker of poor outcome in patients with A/H1N1 virus infection. Lung. 2015;193(1):25‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nicolini G, Forini F, Kusmic C, Iervasi G, Balzan S. Angiopoietin 2 signal complexity in cardiovascular disease and cancer. Life Sci. 2019;15(239):117080. [DOI] [PubMed] [Google Scholar]
- 22.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang N, Li D, Wang X, Sun Z. Abnormal Coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844‐847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muller MP, Tomlinson G, Marrie TJ, et al. Can routine laboratory tests discriminate between severe acute respiratory syndrome and other causes of community‐acquired pneumonia? Clin Infect Dis. 2005;40:1079‐1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan Li, Wang Q, Zhang D, et al. Lymphopenia predicts disease severity of COVID‐19: a descriptive and predictive study. Signal Transduction and Targeted Therapy. 2020;5(1). 10.1038/s41392-020-0148-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan C, Huang Y, Shi F, et al. C‐reactive protein correlates with computed tomographic findings and predicts severe COVID‐19 early. J Med Virol. 2020;92(7):856‐862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henry BM, de Oliveira MHS, Benoit S, Plebani M, Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID‐19): a meta‐analysis. Clin Chem Lam Med. 2020;58(7):1021‐1028. [DOI] [PubMed] [Google Scholar]
- 28.Leth‐Larsen R, Nordenbaek C, Tornoe I, et al. Surfactant protein D (SP‐D) serum levels in patients with community‐acquired pneumonia. Clin Immunol. 2003;108(1):29‐37. [DOI] [PubMed] [Google Scholar]
- 29.Kerget B, Kerget F, Koçak AO, et al. Are serum interleukin 6 and surfactant protein D levels associated with the clinical course of COVID‐19? Lung. 2020;12:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smadja DM, Guerin CL, Chocron R, et al. Angiopoietin‐2 as a marker of endothelial activation is a good predictor factor for intensive care unit admission of COVID‐19 patients. Angiogenesis. 2020;23(4):611‐620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li F, Yin R, Guo Q. Circulating angiopoietin‐2 and the risk of mortality in patients with acute respiratory distress syndrome: a systematic review and meta‐analysis of 10 prospective cohort studies. Ther Adv Respir Dis. 2020;14:1753466620905274. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


