Highlights
-
•
Inflammatory myofibroblastic tumor of the uterus (UMT) is a rare but aggressive malignancy that is often misdiagnosed.
-
•
Immunohistochemistry (IHC) and next generation sequencing (NGS) are essential for accurate diagnosis of UMT.
-
•
We report good therapeutic activity of ALK inhibition in UMT harboring ALK fusions, with responses lasting ≥12 months.
-
•
After disease progression or intolerance to first-generation ALK inhibitors, second-generation inhibitors were beneficial.
-
•
Support for use of second- and third-generation ALK inhibitors should be considered in tumors with ALK rearrangements.
Keywords: Sarcoma, Uterine neoplasms, ALK, Tyrosine Kinase inhibitor (TKI), Inflammatory Myofibroblastic Tumor, Leiomyosarcoma
Abstract
Inflammatory myofibroblastic tumor (IMT) of the uterus is a rare but aggressive malignancy that is often misdiagnosed. Approximately 50% of uterine IMTs (UMT) harbor rearrangements involving the ALK gene on chromosome 2p23 with subsequent overexpression of the ALK protein. Molecular characterization and wider availability of immunohistochemistry (IHC) and next generation sequencing (NGS) have improved clinical recognition and accurate diagnosis of UMT. The discovery of ALK fusions as a genomic driver led to the FDA approval of ALK inhibitors in ALK-altered lung cancers, but there are limited data to date on the spectrum of ALK fusions or patterns of response and resistance to ALK inhibitors in ALK-altered UMT. In this report we describe the genomic and histopathological characteristics and the response to ALK-targeted therapy in four patients with UMT. In all four patients, clinical activity of ALK inhibition was observed, with durable responses lasting 12 months or more. Moreover, three patients derived benefit from a second-generation ALK inhibitor after progression of disease or intolerance to the first-generation inhibitor crizotinib. Our report advocates for consideration of expanding the current National Comprehensive Cancer Network (NCCN) guidelines to include later-generation ALK inhibitors for the treatment of ALK-rearranged UMTs.
1. Introduction
Inflammatory myofibroblastic tumor (IMT) is a mesenchymal neoplasm that often occurs in the lung or soft tissues of the abdomen, pelvis, and retroperitoneum (Gleason and Hornick, 2008). Uterine IMT (UMT) is rare (Bennett et al., 2017), but given the increased availability of immunohistochemistry (IHC) and next generation sequencing (NGS), recognition of this neoplasm has increased. Approximately 50% of IMT harbor rearrangements involving the anaplastic lymphoma kinase (ALK) gene on chromosome 2p23, which encodes a transmembrane receptor tyrosine kinase (Antonescu, et al., 2015). These rearrangements result in fusion of the 3′ kinase portion of ALK to the 5′ portion of a partner gene, and are an established oncogenic driver in non-small cell lung cancer (NSCLC) (Katayama et al., 2015) and other solid tumors, including IMT. Activation of the ALK signaling pathway may represent a critical early step in neoplastic transformation in ALK fusion-positive cancers (Davare and Tognon, 2015).
The discovery of ALK fusion as a genomic driver in IMT has had an impact on clinical care. One case report noted a 6-month response to crizotinib in a patient with an abdominal IMT (Butrynski et al., 2010), leading the NCCN to designate crizotinib and ceritinib as preferred regimens, with activity in IMT with ALK translocations (National Comprehensive Cancer Network, 2020). However, few reports describe the spectrum of ALK fusions seen in UMT and the patterns of response and resistance to ALK inhibitors. We report a case series of four patients (Table 1) with UMT harboring ALK fusions, treated with ALK-targeted therapy.
Table 1.
Summary of IHC, ALK fusion status, and previous treatment of each patient case.
| IHC Staining |
ALK Evaluation |
Prior Therapy |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Case Number | Desmin | SMA | Estrogen Receptor | Progesterone Receptor | ALK IHC | ALK FISH | Fusion | No. of Prior treatments prior to ALK targeted therapy | Prior Chemo or TKI | Prior Endocrine therapy |
| 1 | Negative | Positive | Positive | Positive | Equivocal | Not performed | FN1-ALK | 1 | 0 | Exemestane |
| 2 | Positive | Positive | Positive | Positive | Positive | Positive | TNS1-ALK | 0 | 0 | 0 |
| 3 | Negative | Positive | Positive | Negative | Positive | Positive | LBH-ALK | 1 | Gemcitabine/Docetaxel | 0 |
| 4 | Positive | Not performed | Not performed | Not performed | Positive | Not performed | IGFBP5 -ALK | 3 | Doxorubicin/olaratumab Gemcitabine/docetaxel Pazopanib |
0 |
2. Cases
2.1. Case 1
A 58-year-old woman presented with Stage 1 uterine myxoid leiomyosarcoma (LMS). The tumor demonstrated spindle cells with mild atypia, abundant myxoid matrix, brisk mitotic activity, tumor necrosis, and rare inflammatory cells (Table 1; Fig. 1A). After two years of observation, she experienced her first recurrence: a right pelvic sidewall mass that was resected and demonstrated SMA, ER, and PR expression, no desmin, and equivocal ALK staining. Tissue from the recurrent tumor was sent for the Memorial Sloan Kettering (MSK)-Solid Fusion Assay (SFA) (Zheng et al., 2014, Benayed et al., 2019); a customized targeted RNA-based NGS panel using Archer Anchored Multiplex PCR (AMP™) technology to detect gene fusions and oncogenic isoforms in 62 genes; an FN1-ALK fusion was detected. The ALK rearrangement and negative desmin staining prompted reclassification of the tumor as IMT. Three months later she had a second recurrence at the vaginal apex. She was treated with exemestane until progression of disease. She was then treated with crizotinib and achieved stable disease for 4 months before progression. At this time, her treatment was switched to ceritinib, and she had stable disease for an additional 6 months. Unfortunately, the patient’s disease again progressed. She was then treated with gemcitabine, but 4 months later she developed further progression and died of disease.
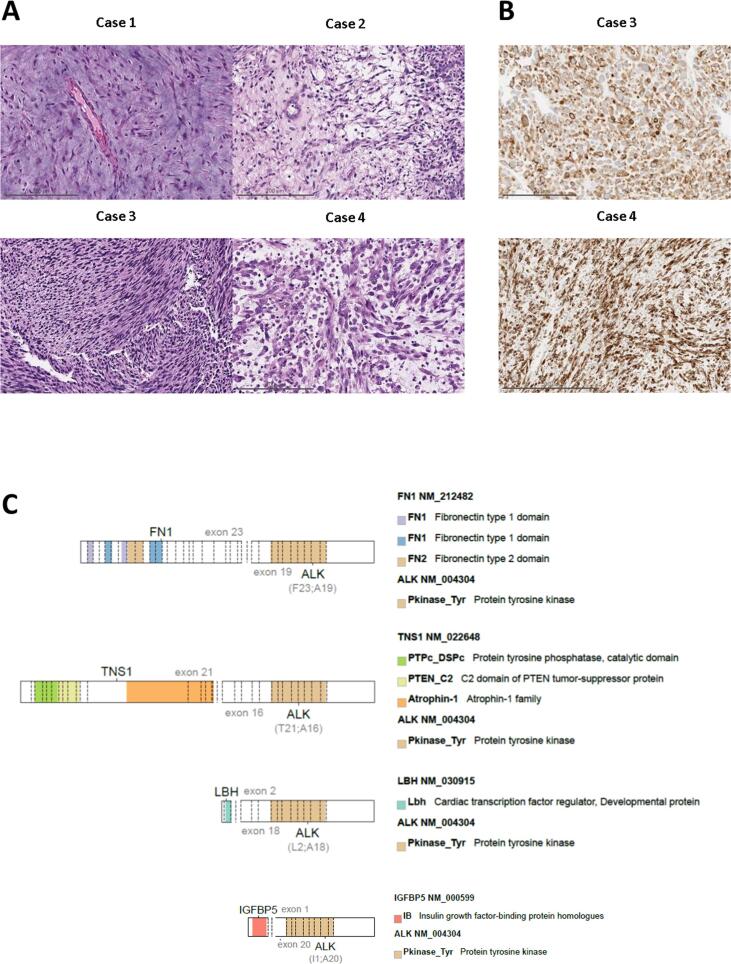
Fig. 1.
Uterine mesenchymal tumors harboring ALK fusions: Histology, ALK expression and ALK fusion status. (Panel A) All tumors consist of spindle cells that demonstrate variable nuclear atypia, myxoid matrix, and associated inflammation across cases. (Panel B) ALK IHC shows strong, diffuse cytoplasmic staining. (Panel C) The full-length coding sequence of the tyrosine kinase domain of ALK is retained in all tumors.
2.2. Case 2
A 68-year-old woman presented with pelvic pain and a pelvic mass. She underwent a total laparoscopic hysterectomy and bilateral salpingo-oophorectomy (TLH/BSO) at an outside hospital, where the histologic diagnosis was atypical leiomyoma. Eighteen months later, she developed abdominal distention and pain. A CT scan demonstrated a large heterogenous pelvic mass and peritoneal implants. She underwent incomplete laparoscopic resection of the pelvic mass, and subsequently presented to our institution. Histologic exam of both the primary and the recurrent tumor demonstrated spindle cells with moderate atypia, focal myxoid change, brisk mitotic activity, rare inflammatory cells and expressed desmin, SMA, ER, PR, and ALK (Table 1; Fig. 1). ALK rearrangement was detected by fluorescence in situ hybridization (FISH) and confirmed by the MSK-SFA, in which an TNS1-ALK fusion was identified. Both the primary and the recurrent tumor were diagnosed as IMT. She was treated with crizotinib for 3 months, with improvement in cancer-associated pain and symptoms; however, she developed CTCAE grade 2 transaminitis, which persisted despite dose adjustments. Crizotinib was stopped and the patient was started on alectinib. She required a dose reduction for elevated bilirubin and continued alectinib. She achieved objective radiographic response and remained on alectinib for 12 months before the disease progressed. She was subsequently treated with ceritinib but her disease progressed after 2 months; she then received lorlatinib but had clinical progression after 1 month. She was started on gemcitabine and docetaxel, but the disease progressed rapidly and she died of disease-related complications.
2.3. Case 3
A 61-year-old woman was found to have a para-tracheal mass on routine chest x-ray. A CT scan showed the chest mass and a large heterogeneously enhancing mass in the upper uterine body/fundus. An endometrial biopsy demonstrated spindle cells with severe atypia, low mitotic index, and ALK, SMA, ER expression without desmin or PR staining (Table 1; Fig. 1). FISH detected an ALK rearrangement, but no fusions were detected by the MSK-SFA. The patient was treated with gemcitabine and docetaxel but had progression of disease. Biopsy of a lung mass demonstrated histology similar to the endometrial biopsy. An LBH-ALK fusion was detected by MSK-SFA, supporting a diagnosis of myofibroblastic sarcoma. She was treated with crizotinib and had stable disease for 30 months. After that her disease progressed, and treatment was changed to ceritinib. She had stable disease for 6 months before further progression. She was subsequently treated with liposomal doxorubicin with progression of disease after 2 cycles. Treatment was changed to a third-generation ALK-inhibitor, lorlatinib. After only 1 week of treatment, CT scans showed a dramatic radiographic response in lung and pleural metastases. Unfortunately, the patient’s co-morbidities of heart failure and asthma, and the development of lorlatinib-related central nervous system toxicity, necessitated discontinuation of lorlatinib. Three months later, patient died of progressive disease.
2.4. Case 4
A 70-year-old woman presented with postmenopausal bleeding and enlarging fibroid uterus. She underwent TLH/BSO, omentectomy, and pelvic node dissection at an outside institution and was diagnosed with uterine myxoid LMS. She received multiples lines of chemotherapy (Table 1) as well as pazopanib, with progression of disease. She was then seen at our institution, where review of the primary uterine tumor demonstrated markedly atypical epithelioid and spindle cells with myxoid matrix, inflammation, and desmin and ALK expression, consistent with IMT (Table 1; Fig. 1). FISH confirmed an ALK rearrangement, and an IGFBP5-ALK fusion was detected by the MSK-SFA. She was started on ceritinib with radiologic response, and greater than 24 months since initiation, remains on therapy to date.
3. Discussion
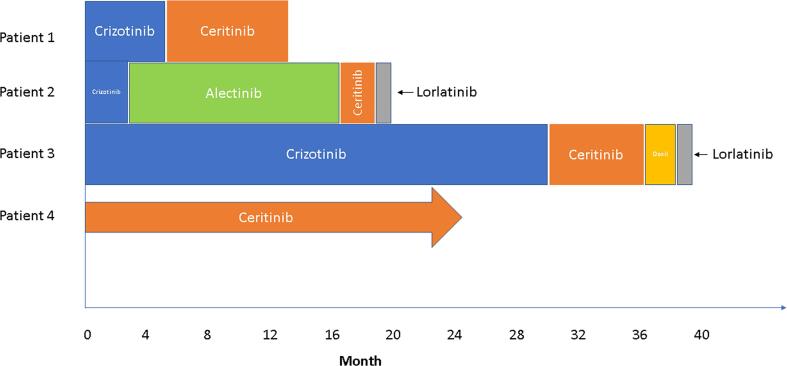
In this report, we demonstrate the clinical activity of ALK inhibition in four patients with UMT harboring ALK fusions. Responses were durable, lasting 12 months or more; moreover, three patients derived benefit from a second-generation ALK inhibitor after progression of disease or intolerance to the first-generation inhibitor crizotinib (Fig. 2). To date, the FDA approval of ALK inhibitors has been confined to the treatment of ALK-altered NSCLC, and only crizotinib and ceritinib are listed by the NCCN as treatment for extrapulmonary sarcomas with ALK fusions (National Comprehensive Cancer Network, 2020).
Fig. 2.
Response to ALK inhibitors in uterine mesenchymal tumors harboring ALK fusions.
Three of our patients were initially diagnosed with a smooth muscle tumor (atypical leiomyoma or myxoid LMS). All tumors demonstrated myxoid matrix, with two showing myogenic differentiation. Inflammation was prominent in only one case. In all patients, IHC, FISH, and/or NGS detected ALK over-expression and subsequent oncogenic ALK fusions, permitting tumor reclassification and guiding treatment decisions. In 80 cases of LMS from the Cancer Genome Atlas Research Network's comprehensive characterization of soft tissue sarcomas, no oncogenic kinase fusions were reported (Cancer Genome Atlas Research Network, 2017). However, in a set of 967 sarcomas sequenced by Foundation Medicine, including 5 uterine LMS with ALK fusions, multiple targetable kinase fusions were reported (Elvin, et al., 2014). Our report suggests that myxoid UMT, with or without myogenic differentiation, may be enriched for ALK fusions. Given the profound clinical benefit of ALK inhibition, a low threshold for ALK fusion screening in myxoid UMT is recommended.
Historically, there has been good concordance between ALK gene rearrangements and overexpression by IHC (Cook et al., 2001). In this report, three tumors demonstrated definitive ALK immunoexpression (Table 1). An algorithmic approach to molecular diagnosis in uterine mesenchymal tumors can be applied, whereby an ALK IHC-positive tumor undergoes confirmatory ALK FISH or NGS. ALK IHC can also determine whether the ALK fusion is functionally expressed.
Of note, the ALK fusion partners seen in UMT (Fig. 1) differ from those described in the NSCLC literature (Katayama et al., 2015). In NSCLC, EML4-ALK fusions predominate. Across a wide range of cancers, several other fusion partners have been identified that serve to constitutively activate ALK, and EML4 involvement is less frequent (Lawrence et al., 2000); in IMT, these partners include TPM3, TPM4, and FN1. IGFBP5 and TNS1 have also previously been reported in UMT but are less common in NSCLC (Bennett et al., 2017, Haimes et al., 2017). FN1 and LBH have been characterized in both lung and UMTs. The activity of ALK fusions may differ depending on the fusion partner, with some partners driving a higher level of expression of the oncogenic fusion kinase than others. This differential expression may impact sensitivity to a moderate ALK inhibitor like crizotinib but may be less relevant in the setting of more potent second- and third-generation ALK inhibitors. The impact of fusion partners on drug efficacy remains under investigation (Chuang et al., 2019).
In conclusion, patients with myxoid UMT harboring ALK rearrangement may derive durable benefit from treatment with ALK inhibitors. ALK IHC with confirmatory NGS is recommended in the setting of any myxoid uterine mesenchymal neoplasm with or without myogenic differentiation. Given the rarity of this disease, the current report includes only a small number of patients. Nevertheless, it is notable that several of the patients reported herein benefited from second-generation ALK inhibitors after progression on crizotinib. Consideration should be given to a tumor-agnostic approach to drug development as well as guideline support for use of second- and third-generation ALK inhibitors in tumors with ALK rearrangements.
4. Consent
Authors obtained informed consent to publish information and/or images from each participant included in this case series.
Funding
The study was supported in part by the NIH/NCI Cancer Center Support Grant P30 CA008748.
CRediT authorship contribution statement
Chrisann Kyi: Investigation, Writing – review & editing. Claire F. Friedman: Investigation, Writing – review & editing. Jennifer J. Mueller: Investigation, Writing – review & editing. Ryma Benayed: Formal analysis, Investigation, Resources. Marc Ladanyi: Formal analysis, Investigation, Resources. Maria Arcila: Formal analysis, Investigation, Writing – review & editing. Soo Ryum Yang: Formal analysis, Visualization, Writing – review & editing. Martee L. Hensley: Conceptualization, Writing – review & editing, Supervision. Sarah Chiang: Conceptualization, Writing – review & editing, Supervision.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: ‘CFF reports personal/consultancy fees from AstraZeneca, as well as participation in scientific steering committees (compensation waived) for Merck and Genentech. These are outside the scope of the submitted work. She also reports institutional research funding from Genentech, Merck, Bristol Myers Squibb, and AstraZeneca. CK reports research funding from Bristol Myers Squibb, Merus, and Gritstone Oncology, outside the scope of the submitted work. MLH reports that a family member is employed by Sanofi. She reports personal fees from GSK, Tesaro, and Lilly (advisory boards), personal fees from Research to Practice (CME faculty speaker), and author royalties from Up to Date. These are outside the scope of the submitted work. ML reports personal fees from Merck, AstraZeneca, Bristol Myers Squibb, Takeda, Lilly Oncology, Bayer, ADC Therapeutics, Paige AI; and research support from LOXO Oncology, Merus, Helsinn Therapeutics. These are outside the scope of the submitted work. MA reports personal fees (speakers fees, consultancy) from Biocartis, invivoscribe, Janssen Global, Bristol Myers Squibb, AstraZeneca Pharmaceuticals, outside the scope of the submitted work’.
Acknowledgements
The authors would like to thank the patients who were included in this study.
Contributor Information
Chrisann Kyi, Email: kyic@mskcc.org.
Claire F. Friedman, Email: friedmac@mskcc.org.
References
- Antonescu C.R. Molecular characterization of inflammatory myofibroblastic tumors with frequent ALK and ROS1 gene fusions and rare novel RET rearrangement. Am. J. Surg. Pathol. 2015;39(7) doi: 10.1097/PAS.0000000000000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benayed R. High yield of RNA sequencing for targetable kinase fusions in lung adenocarcinomas with no mitogenic driver alteration detected by DNA sequencing and low tumor mutation burden. Clin. Cancer Res. 2019;25(15):4712–4722. doi: 10.1158/1078-0432.CCR-19-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett J.A. Inflammatory myofibroblastic tumor of the uterus: a clinicopathological, immunohistochemical, and molecular analysis of 13 cases highlighting their broad morphologic spectrum. Mod. Pathol. 2017;30(10):1489–1503. doi: 10.1038/modpathol.2017.69. [DOI] [PubMed] [Google Scholar]
- Butrynski J.E. Crizotinib in ALK-rearranged inflammatory myofibroblastic tumor. N. Engl. J. Med. 2010;363(18):1727–1733. doi: 10.1056/NEJMoa1007056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancer Genome Atlas Research Network Comprehensive and integrated genomic characterization of adult soft tissue sarcomas. Cell. 2017;171(4) doi: 10.1016/j.cell.2017.10.014. 950–965 e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang Y.-C. Molecular modeling of ALK L1198F and/or G1202R mutations to determine differential crizotinib sensitivity. Sci. Rep. 2019;9(1):11390. doi: 10.1038/s41598-019-46825-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J.R. Anaplastic lymphoma kinase (ALK) expression in the inflammatory myofibroblastic tumor: a comparative immunohistochemical study. Am. J. Surg. Pathol. 2001;25(11):1364–1371. doi: 10.1097/00000478-200111000-00003. [DOI] [PubMed] [Google Scholar]
- Davare M.A., Tognon C.E. Detecting and targetting oncogenic fusion proteins in the genomic era. Biol. Cell. 2015;107(5):111–129. doi: 10.1111/boc.201400096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elvin J.A. Genomic profiling of uterine leiomyosarcomas reveal frequent alterations in Akt/mammalian target of rapamycin (mTOR) pathway genes and other actionable genomic abnormalities linked to targeted therapies. Eur. J. Cancer. 2014;50 104–104. [Google Scholar]
- Gleason B.C., Hornick J.L. Inflammatory myofibroblastic tumours: where are we now? J. Clin. Pathol. 2008;61(4):428–437. doi: 10.1136/jcp.2007.049387. [DOI] [PubMed] [Google Scholar]
- Haimes J.D. Uterine inflammatory myofibroblastic tumors frequently harbor ALK fusions with IGFBP5 and THBS1. Am. J. Surg. Pathol. 2017;41(6):773–780. doi: 10.1097/PAS.0000000000000801. [DOI] [PubMed] [Google Scholar]
- Katayama R., Lovly C.M., Shaw A.T. Therapeutic targeting of anaplastic lymphoma kinase in lung cancer: a paradigm for precision cancer medicine. Clin. Cancer Res. 2015;21(10):2227–2235. doi: 10.1158/1078-0432.CCR-14-2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence B. TPM3-ALK and TPM4-ALK oncogenes in inflammatory myofibroblastic tumors. Am. J. Pathol. 2000;157(2):377–384. doi: 10.1016/S0002-9440(10)64550-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Comprehensive Cancer Network. Soft Tissue Sarcoma (Version 2.2020). October 29th, 2020]; Available from: https://www.nccn.org/professionals/physician_gls/pdf/sarcoma.pdf.
- Zheng Z. Anchored multiplex PCR for targeted next-generation sequencing. Nat. Med. 2014;20(12):1479–1484. doi: 10.1038/nm.3729. [DOI] [PubMed] [Google Scholar]