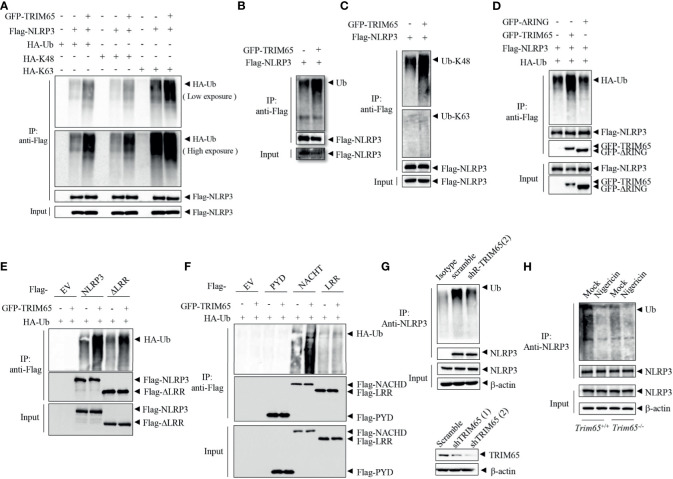
Figure 3.
TRIM65 promotes NLRP3 lys48- and lys63- linked ubiquitination. (A) Flag-NLRP3 and GFP-TRIM65 were cotransfected with HA-Ubiquitin, HA-Ubiquitin (K48-linked only), or HA-Ubiquitin (K63-linked only) in HEK-293T cells. The ubiquitination of NLRP3 was analyzed by immunoprecipitation and western blotting. (B, C) Flag-NLRP3 was cotransfected with GFP-TRIM65 in HEK- 293T cells. The overall ubiquitination (B), K48-linked ubiquitination (C) and K63-linked ubiquitination (C) of NLRP3 were analyzed by immunoprecipitation and western blotting. (D) Flag-NLRP3 was cotransfected with GFP-TRIM65 or GFP-TRIM65 lacking the RING domain in HEK-293T cells. The ubiquitination of NLRP3 was analyzed by immunoprecipitation and western blotting. (E, F) Full-length Flag-NLRP3 and NLRP3 truncation mutants were individually cotransfected with GFP-TRIM65 in HEK-293T cells. Ubiquitination of NLRP3 or its truncation mutants was analyzed by immunoprecipitation and western blotting. (G) Endogenous ubiquitination of NLRP3 in PMA-differentiated and LPS-primed THP-1 cells stably expressing scrambled shRNA or shRNA targeting TRIM65 mRNA was analyzed by immunoprecipitation and western blotting. (H) LPS-primed BMDMs from wild-type or Trim65-/- mice were stimulated with nigericin, and the endogenous ubiquitination of NLRP3 was analyzed by immunoprecipitation and western blotting. Data are representative of two or three independent experiments. EV, empty vector.