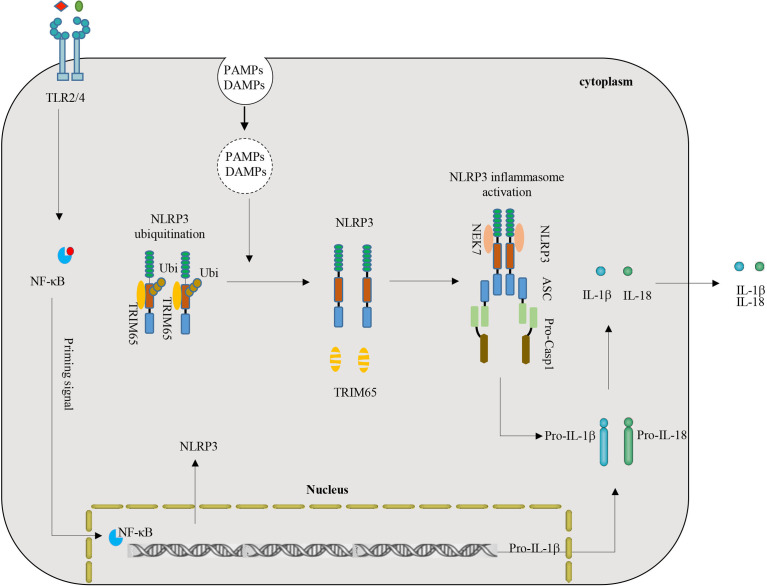
Figure 6.
Model of the function and mechanism of TRIM65-mediated inhibition of NLRP3 inflammasome activation. TRIM65 directly binds NLRP3 and promotes K48- and K63- linked ubiquitination, which is critical for the inhibition of NLRP3 inflammasome activation in resting and priming macrophages. With stimulation of priming signals (for example LPS-TLR4-Myd88-NF-κB) and PAMPs or DAMPs (such as MSU, ATP, R837, Nigericin, etc.), TRIM65 significantly decreased, follow by attenuated ubiquitination of NLRP3, strengthened NLRP3-NEK7 interaction, assembled and activated NLRP3 inflammasome. (PAMPs, pathogen-associated molecular patterns; DAMPs, danger-associated molecular patterns).