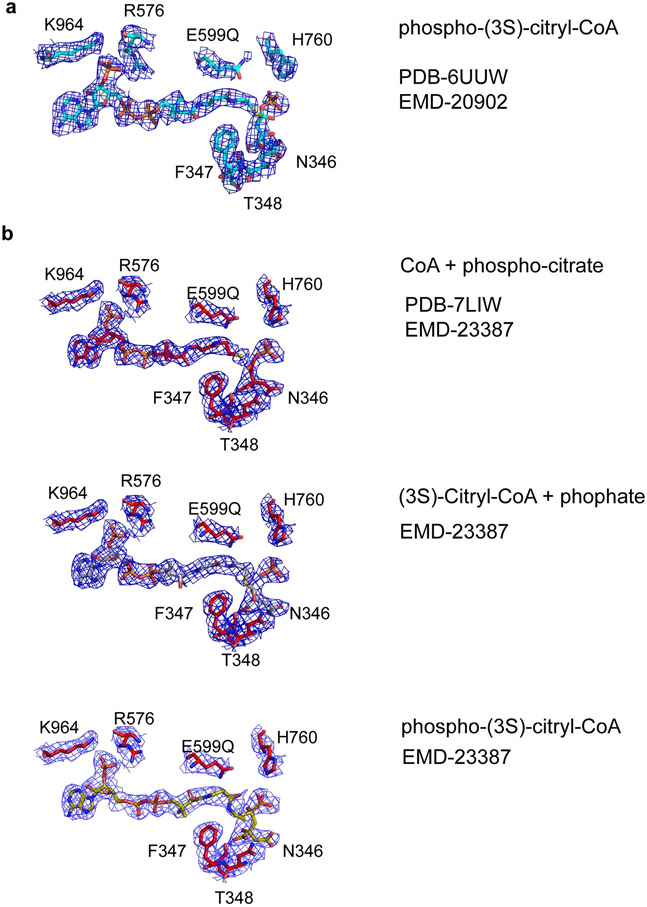
Figure 2.
Cryo-EM density of a reaction intermediate in the ACLY active site. (a) Cryo-EM density (2.5 σ) corresponding to modeled phosphor-(3S)-citryl-CoA in the ASH domain of the ACLY-E599Q–ATP–citrate–CoA-D2 structure (PDB- 6UUW/EMD-20902) reported in Wei et al.2. (b) Cryo-EM density (3.5 σ) corresponding to the reaction intermediate in the ASH domain after non-uniform refinement together with particle symmetry expansion, particle subtraction and local refinement in Cryosparc (PDB-7LIW/EMD-23387) 4,5. While the cryo-EM density can be best modeled as CoA + phospho-citrate (top, PDB-7LIW), at the current resolution we cannot exclude the additional presence of phosphate + (3S)-citryl-CoA (middle), and phospho-(3S)-citryl-CoA (bottom). The figure was made in Pymol. Important contacting residues, and catalytic E599, with their corresponding cryo-EM densities are shown for reference.