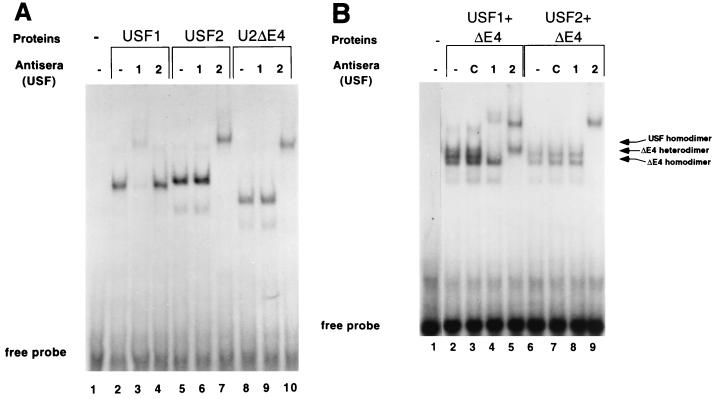
FIG. 5.
U2ΔE4 can bind the class I E box. Equivalent amounts of in vitro-translated USF proteins (determined by SDS-PAGE of 35S-labeled proteins; data not shown) were added to end-labeled class I E-box probe, and binding reactions were analyzed as described in the legend to Fig. 2 and in Materials and Methods. (A) Homodimer of U2ΔE4 can bind the class I E box. Lane 1, probe alone; lanes 2 to 4, USF1; lanes 5 to 7, USF2; lanes 8 to 10, U2ΔE4. Antisera against USF1 or USF2 were included to verify the identities of the translated proteins and demonstrate the specificity of the individual antisera. Lanes 3, 6, and 9 contain the anti-USF1 antiserum. Lanes 4, 7, and 10 contain the anti-USF2 antiserum. Specific supershifted complexes are present in lanes 3 (USF1), 7 (USF2), and 10 (U2ΔE4). Note the slower mobility of USF2 (due to its higher molecular weight) than that of USF1 and the faster mobility of U2ΔE4. (B) U2ΔE4 can generate class I E box-binding dimers with USF1 and USF2. U2ΔE4 was cotranslated with either USF1 or USF2, and the specific binding complexes generated were examined by antibody supershift analyses. Lane 1, probe alone; lanes 2 to 5, U2ΔE4 and USF1; lanes 6 to 9, U2ΔE4 and USF2. The antiserum against either USF2 or USF1 was added to distinguish homodimers from heterodimers. Control (C) antisera against the p50 subunit of NF-κB was added to the binding reaction mixtures shown in lanes 3 and 7. The antiserum against either USF1 or USF2 was added to the binding reaction mixtures shown in lanes 4 and 8 and 5 and 9, respectively.