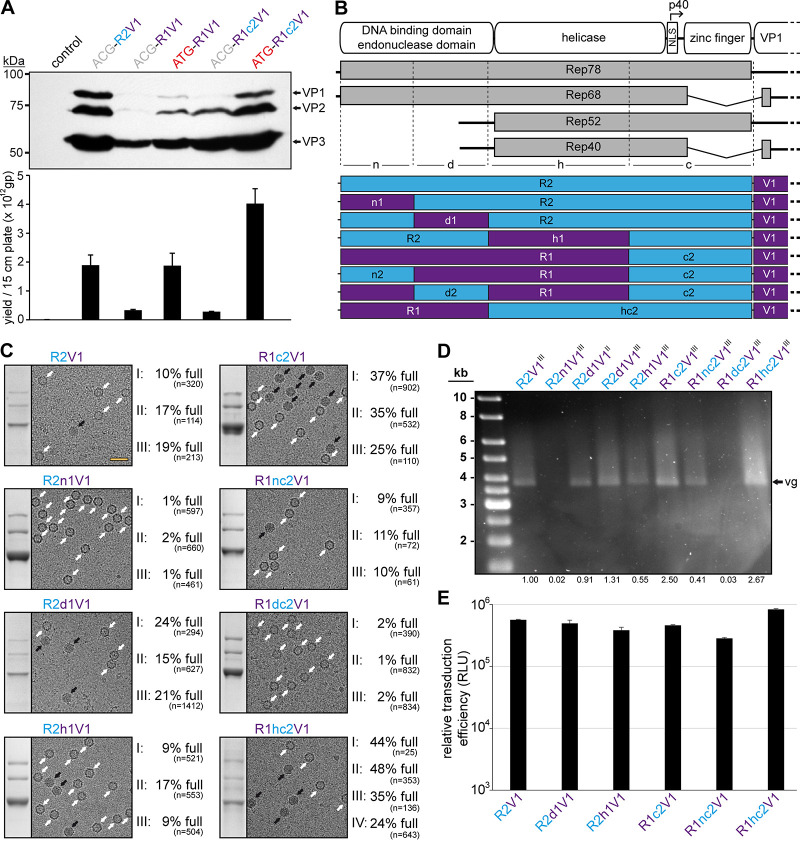
FIG 5.
AAV1/2 Rep hybrids improve AAV1 genome packaging efficiency. (A) Analysis of VP expression by Western blotting and the AAV1 vector yield by qPCR after transfection of the constructs in HEK293 cells. The Western blot was probed with MAb B1. The individual VPs are indicated. (B) Schematic depiction of the Rep protein with its main domains. The approximate position of the p40 promoter in the rep gene is indicated. Below, the p5 and p19 transcripts are shown with the translated regions for Rep78, Rep68, Rep52, and Rep40. Rep has been subdivided into a N-terminal domain (n), the DNA-binding domain (d), the helicase domain (h), and a C-terminal domain (c). The generated constructs containing different domains from AAV1 and AAV2 are depicted below. rep gene fragments derived from AAV1 are colored in purple and those from AAV2 are colored in blue. (C) Analysis of the AVB-purified AAV1 vector preparations. Sections of SDS-PAGEs containing VP1, VP2, and VP3 and representative example cryo-EM micrographs are shown for each Rep hybrid. White arrows point to empty capsids (light appearance), and black arrows point to full capsids (dark appearance). The determined percentages of full capsids of at least three independently produced and purified AAV1 vector preparations are displayed with the total particle count of all micrographs collected for the individual sample. Scale bar (shown in R2V1 micrograph), 50 nm. (D) Alkaline gel electrophoresis of the AAV1 vector preparations. The capsid amount loaded is the same for all samples, based on the ELISA titer. The size of the packaged vector genome (vg) is ∼3.9 kb. The intensities of the vector genome bands were quantified using ImageJ and normalized to R2V1. (E) Analysis of the transduction efficiency of the AAV1 vectors produced with different Rep hybrids in HEK293 cells.