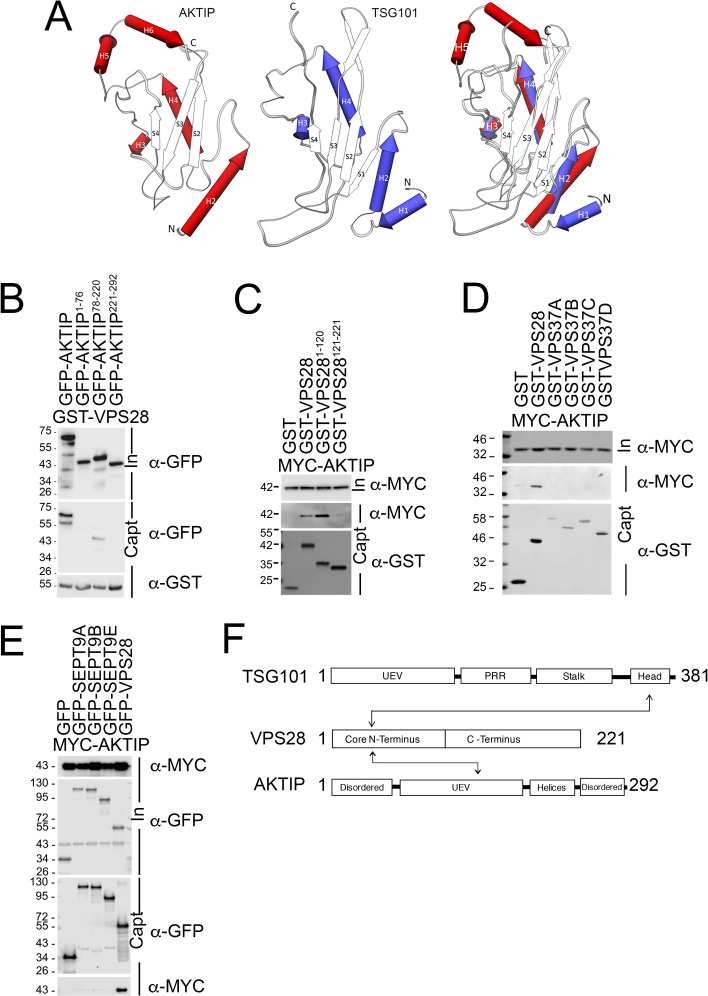
Fig 3. The central region of AKTIP interacts with the N-terminus of VPS28.
(A) Superimposing of AKTIP model on TSG101 X-ray solved structure highlights similarities in the central region and two main different elements outside of it. Namely, the AKTIP central UEV domain presents two C-terminal helices (H5 and H6), absent in TSG101; TSG101 contains two N-terminal helices (H1 and H2), while AKTIP only one (H2). (B-C) Western blotting showing that central UEV region of AKTIP interacts with GST-VPS28 (B) and that AKTIP interacts with N-terminal domain of VPS28 (C). Cells were transfected with plasmids encoding the indicated fusion proteins and the AKTIP protein N-terminal (1–76 aa, NT), central domain (78–220 aa), C-terminal (221–292 aa) fragments. Purified VPS28-GST, or N-terminal (1–120 aa, NT) VPS28, or C-terminal (121-221aa, CT) or GST alone were used to pull down interacting proteins; cell lysates and glutathione-bound fractions were then analyzed with GST, GFP or MYC antisera. GST-pull downs were repeated three times. (D) Western blotting showing that AKTIP does not interact with VPS37 A to D isotypes and confirming its interaction with VPS28. Cells were transfected with plasmids encoding the indicated fusion proteins. Purified VPS28-GST or VPS37(A-D)-GST or GST alone were used to pull down interacting proteins; cell lysates and glutathione-bound fractions were then analyzed with MYC antisera. GST-pull downs were repeated two times. (E) Western blotting showing that AKTIP does not interact with SEPT9 (A, B and E) and confirming its interaction with VPS28. Cells were transfected with the indicated fusion proteins. Purified SEPT9 (isoforms A, B and E)-GFP, VPS28-GFP or GFP alone were used to trap interacting proteins; then cell lysates and GFP-trapped fractions were analyzed with MYC antisera. GFP-TRAP were repeated three times. (F) Schematic representation of the interacting regions of AKTIP and TSG101 with VPS28. UEV (Ubiquitin E2 variant domain); TSG101 PRR (Proline Rich Region).