Scheme 5. Synthetic Routes of Compounds 25a–ia.
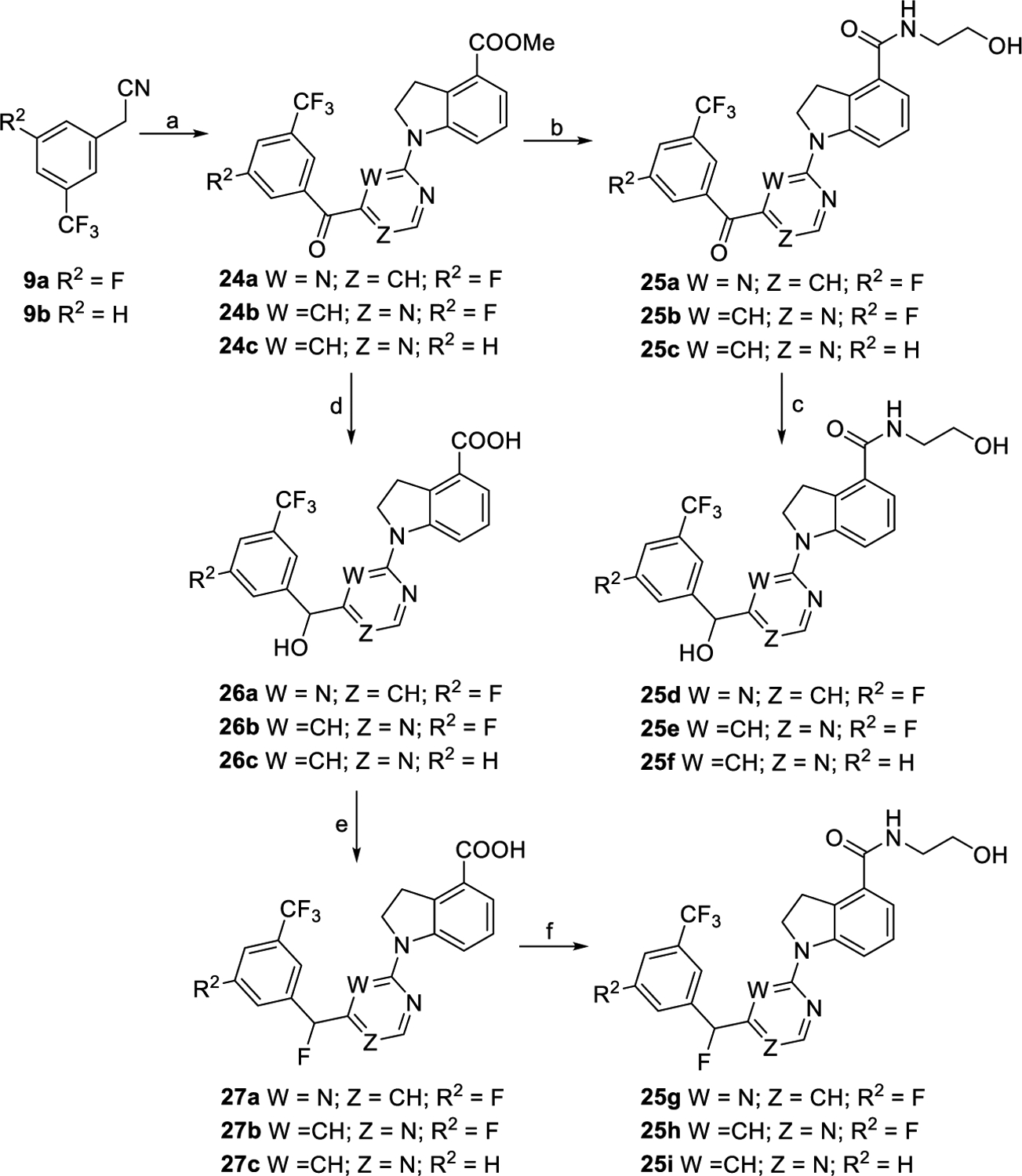
aReagents and conditions: (a) For 24a, (i) 7a, NaH, DMF, 0 °C to rt., and 2 h; (ii) mCPBA, 0 °C to rt., 10 min, and 47% for two steps; (iii) 6, XantPhos, Cs2CO3, Pd(OAc)2, 1,4-dioxane, 100 °C, overnight, and 82%; for 24b and 24c, (i) 8c, NaH, DMF, 0 °C to rt., and 2 h; (ii) mCPBA, 0 °C to rt., 10 min, 49% over two steps for 24b, and 79% over two steps for 24c. (b) (i) Con. HCl/AcOH/H2O, reflux, and overnight; (ii) NH2CH2CH2OH, EDCI, DMAP, DMF, rt., overnight, and 63–77% for two steps. (c) NaBH4, DMF/MeOH, 0 °C to rt., 30 min, and 68–90%. (d) (i) NaBH4, DMF/MeOH, 0 °C to rt., and 30 min; (ii) con. HCl/AcOH/H2O, reflux, overnight, and 78–90%. (e) (i) DAST, CH2Cl2, 0 °C to rt., and 10 min; (ii) THF/H2O, reflux, overnight, and 60–76% for two steps. (f) NH2CH2CH2OH, EDCI, DMAP, DMF, rt., overnight, and 68–86%.
