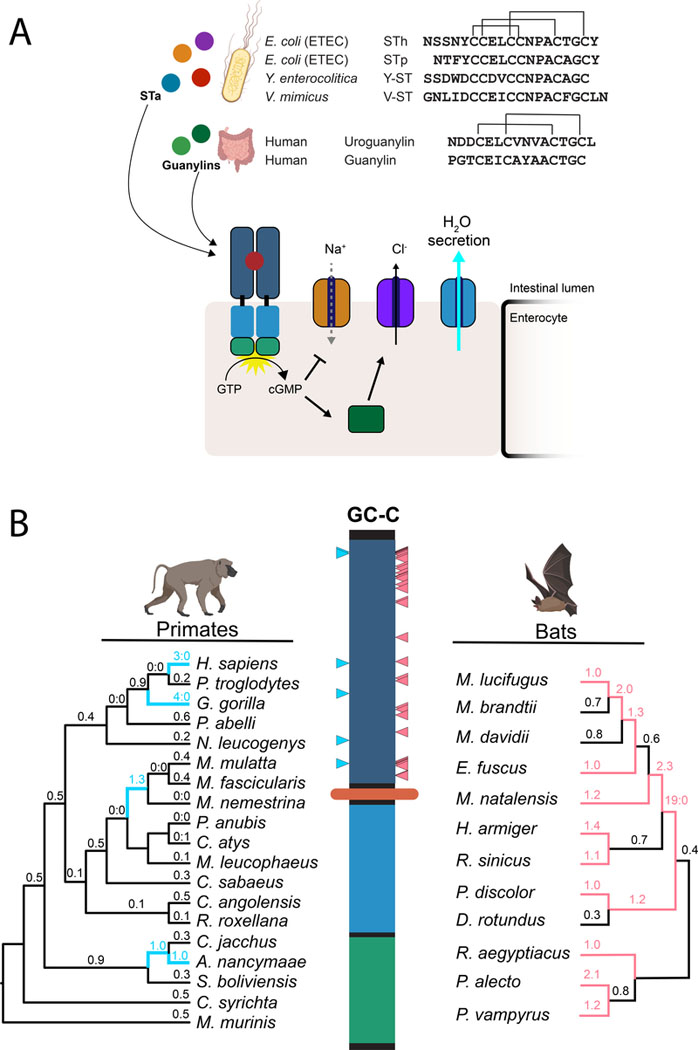
Figure 1: Rapid evolution of the heat-stable enterotoxin receptor in primates and bats.
(A) Amino acid sequences of STa peptides encoded by four human pathogens are shown in comparison to human guanylin and uroguanylin. Disulfide bonds are indicated with connecting lines (top). Schematic of GC-C signaling in intestinal enterocytes. Activation of cGMP synthesis via ligand binding to GC-C results in chloride secretion through CFTR and inhibition of sodium transport via NHE3. Resulting shifts in ion balance facilitate water secretion into the intestinal lumen. Prolonged activation by STa causes excess water secretion and diarrhea (bottom). (B) Evolutionary analysis of GC-C in primates and bats. Phylogenetic trees indicate patterns of rapid evolution in primates (left) and bats (right). Branches with dN/dS >1 are highlighted in blue (primates) or magenta (bats) Codons with evidence of positive selection are indicated on the GC-C primary structure with blue (primates) or magenta (bats) triangles (PAML posterior probability >95%).