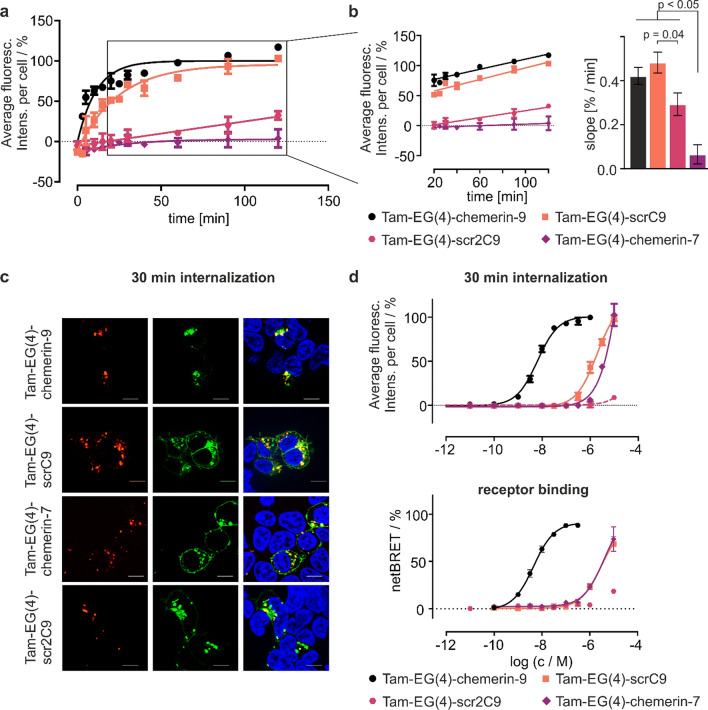
Fig. 7.
Various chemerin-derived peptides are internalized by GPR1. a HEK293 cells stably expressing GPR1 internalize Tam-EG(4)-chemerin-9 and the scrambled peptides Tam-EG(4)-scrC9 and Tam-EG(4)-scr2C9, but not Tam-EG(4)-chemerin-7. Cells were stimulated with 1 µM of the respective peptide; data points represent mean ± SEM from at least two independent experiments performed in duplicates. Intracellular accumulation of the labeled peptides over time was measured using a confocal microscopy high content imaging system. b After 20 min, the concentration of Tam-EG(4)-chemerin-9 and both scrambled peptides linearly increases over time. The slope of the linear regression for Tam-EG(4)-chemerin-7 does not significantly differ from zero, and is significantly smaller than the slope for Tam-EG(4)-chemerin-9, Tam-EG(4)-scrC9, and Tam-EG(4)-scr2C9 (p < 0.05, one-way ANOVA with Tukey’s multiple comparisons post-test). Bars represent mean ± SEM. c After stimulation with 1 μM peptide for 30 min, all internalized peptides co-localize with the GPR1-eYFP, demonstrating a receptor-dependent internalization. d Comparing concentration-dependent internalization in stably transfected HEK293-GPR1-eYFP cells and receptor binding to Nluc-GPR1-eYFP demonstrates that peptides need to bind to the receptor to be internalized