Abstract
Background:
Daily self-weighing (DSW) may be an effective harm-reduction intervention to disrupt continued weight gain. Self-Weighing for Obesity Management in Primary Care (SWOP) is a 24-month randomized controlled trial in 400 adults with obesity (BMI: kg/m2≥30) receiving primary care through a clinical network affiliated with an academic medical center.
Objective:
To test DSW as a potentially scalable way to deter age-related weight gain among primary care patients with obesity.
Methods:
Randomized-controlled trial with two conditions: DSW (instruction to weigh daily and provision of a web-enabled digital scale with graphical weight feedback) or Standard Care (receive a monetary gift card equivalent to value of the scale). Both groups receive standardized weight management educational material. SWOP will test the causal effect of assignment to DSW (Aim 1) and adherence to DSW (Aim 2) on weight (primary outcome) and adoption of weight management practices (secondary outcomes), as well as evaluate the cost-effectiveness of DSW compared to standard care (Aim 3). Findings may inform clinical guidelines for weight management by providing evidence that DSW attenuates continued age-related weight gain among adults with obesity. This trial is registered with ClinicalTrials.gov (NCT04044794).
Keywords: primary care, weight management, harm reduction
1. Introduction
Obesity is the major driver of preventable chronic diseases, imposing annual health care costs of $147 to $210 billion in the United States (US) [1,2]. In the US, an estimated 39.6% of adults have obesity [3], and most adults (98% of men and 92% of women) gradually gain weight over time, averaging 0.53 kg annually [4]. Moreover, adults with class I obesity (body mass index (BMI)= 30.0-34.9 kg/m2) continue to gain substantial weight over an 18-year period, [5] resulting in nearly 50% transitioning into class II (BMI=35.0-39.9 kg/m2) or class III (BMI≥40.0 kg/m2) obesity. This age-related weight gain strongly associates with an increased risk of major chronic diseases (e.g., cardiovascular disease, cancer, type 2 diabetes) and decreased odds of healthy aging [6,7], suggesting that disrupting continued weight gain can reduce adverse outcomes [6].
Multi-component lifestyle interventions for obesity management can achieve meaningful reductions in weight [8–11]. However, such interventions include frequent contact with experienced interventionists [8,12,13], thus limiting both large-scale implementation and implementation in a broader range of localities where residents may otherwise be underserved. Because programs delivered in primary care have widespread reach, health organizations have called for greater involvement of primary care physicians in the provision of weight management services [14,15]. Significant barriers and implementation challenges [16], however, impede the delivery [17–19] and effectiveness [20–23] of weight management programs in primary care, particularly when physicians deliver treatment [20]. Therefore, although primary care may be an optimal setting for the provision of weight management programs, there is need for approaches that are feasible, easily implemented, and sustainable.
Harm reduction approaches (interventions aimed at reducing the negative effects of health behaviors without necessarily extinguishing the problematic health behaviors completely) [24] may be relevant for obesity management in primary care settings by disrupting age-related weight gain. Self-regulation theory suggests that daily self-weighing (DSW; i.e., instruction to weigh daily and monitor weight) may disrupt continued weight gain by fostering greater awareness and accountability, thereby potentially promoting weight management [25]. DSW associates with better weight management in observational studies [25,26] and when implemented alone or within multi-component interventions [25,27–31]. Given this, DSW may be ideal for primary care because of its low-burden and consistency with clinical guidelines recommending patient self-monitoring of health variables (e.g., blood pressure and glucose) [10]. However, DSW’s effect on weight gain prevention, when implemented in primary care among adults with obesity, is unknown. Therefore, the purpose of the Self-Weighing for Obesity Management in Primary Care (SWOP) trial is to test the effects of DSW on weight (primary outcome) and adoption of weight management practices (secondary outcomes) over 24 months.
2. Methods
2.1. Overview of Study Design
SWOP is a 24-month randomized controlled trial that will enroll and randomize adults with obesity to one of two conditions: 1) DSW, in which participants will be instructed to weigh daily using a Wifi-enabled scale providing regular electronic graphical feedback, or 2) standard care (SC), in which participants receive a gift card of equivalent cash value to the digital scale. All participants will receive brief, evidence-based educational materials on dietary modification, physical activity, and behavioral strategies to promote weight management and routine care from their primary care provider (see 2.7 Treatment Conditions and 2.11. Retention). The frequency and quantity of contact time with study staff will be comparable across conditions. Assessments will be completed at baseline and at 6, 12, 18, and 24 months (Figure 1). Due to the COVID-19 pandemic, we were required to modify our data collection procedures and protocols. Table 1 summarizes our original procedures and COVID-related modifications. Here, we describe original study design procedures with highlights of the COVID-19 modifications summarized in section 2.10 COVID-19 modifications below. All protocols were approved by the participating University’s Institutional Review Board and the trial is registered at Clinicaltrials.gov (NCT04044794).
Figure 1.
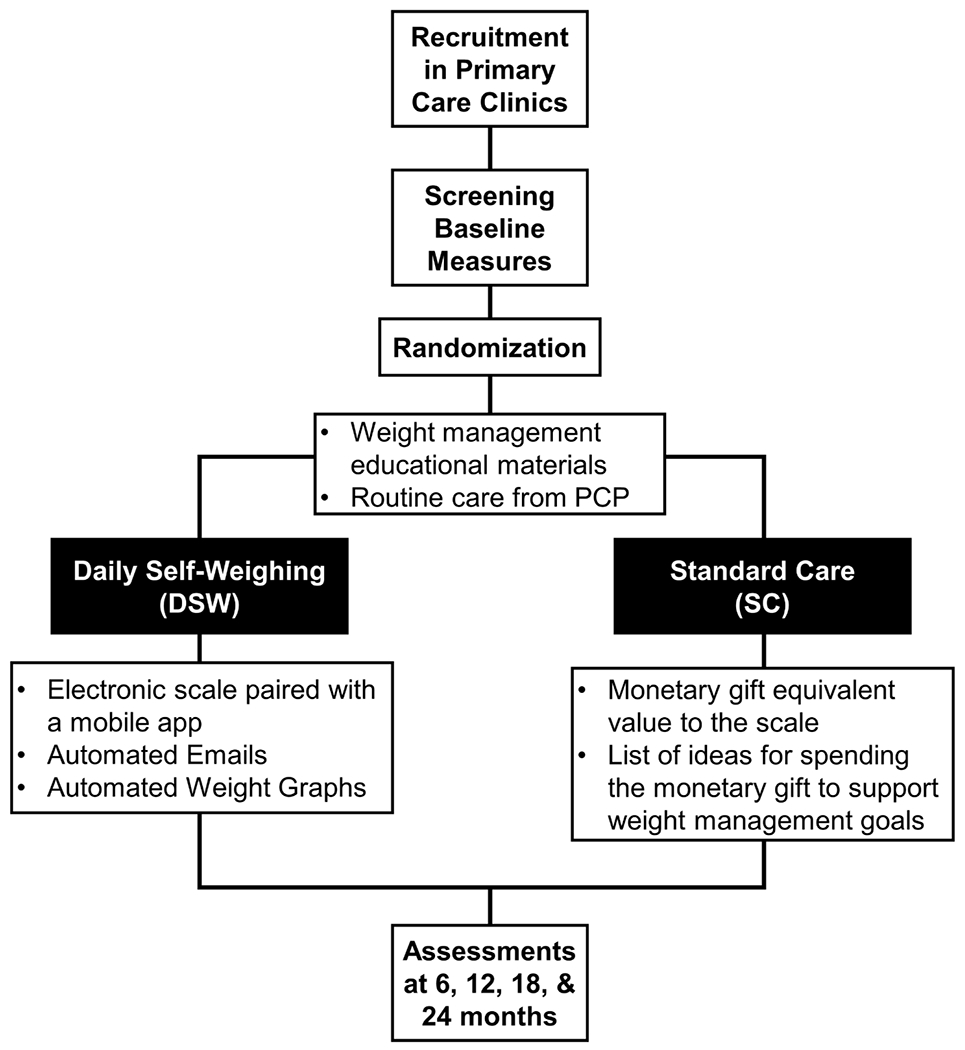
Overview of the study design. DSW, daily self-weighing; SC, standard care
Table 1.
Protocol modifications due to COVID-19
| Original Protocol | COVID-19 Modifications | |
|---|---|---|
| Design | In-person study visits (6 visits) In-person collection of study forms |
A combination of remote (1 visit) and in-person (5 visits) study visits Remote consent and collection of study forms |
| Pre-screening | Pre-screening and brief orientations screened initially by telephone or online (via survey) to determine preliminary eligibility. | Participants expressing interest in the study are pre-screened by phone or online. |
| Screening (Visit 1) | Written orientation materials sent to participant via mail or email, including consent form for review prior to in-person visit In-person visit includes: • Orientation video • Informed consent • Study eligibility confirmation • Scheduled for a baseline visit |
Written orientation materials and orientation video sent to participant via email, including a consent form for review prior to a remote visit Informed consent conducted at remote HIPAA compliant Zoom visit Consent formed signed remotely via Adobe Sign Confirmation/Welcome email sent after receipt of electronic consent; contains a link to fill out baseline questionnaires |
| Baseline assessment (Visit 2) |
In-person visit: Baseline & Randomization Height and weight measured, self-administered questionnaires completed electronically via tablet, then randomized to study group. Standard control: Staff conducts a brief discussion to encourage retention and adherence, provides treatment materials, a monetary gift card containing $60 (the equivalent value to the scale), and a list of ideas for how to spend the monetary gift to support weight management. Daily self-weighing: Staff conducts a brief discussion to encourage retention and adherence, provides treatment materials, and guides participants through scale and app set-up with study-specific parameters. |
Remote data collection: Self-administered questionnaires completed online via provided link. After completion of questionnaires, the participant scheduled for a 15-minute in-person visit to collect anthropometries. In-person visit: Screening, Baseline, and Randomization COVID-19 guidelines implemented: • Screened for symptoms 24h prior to a visit • Sent video and written instructions for in-person visit/COVID-19 protocol • Remain in vehicle until appointment time • Temperature scan and symptom questions at the start of a visit • Visit limited to 15 minutes Height and weight measured then randomized to study group Standard control: Staff conducts a brief discussion to encourage retention and adherence, provides treatment materials, a monetary gift card containing $60 (the equivalent value to the scale), and a list of ideas for how to spend the monetary gift to support weight management. Daily self-weighing: Staff conducts a brief discussion to encourage retention and adherence, provide treatment materials, and guide participants through scale and app set-up with study-specific parameters. Within one week of randomization, staff conducts a follow-up remote visit (via HIPAA-compliant Zoom). |
| 6, 12, 18, and 24 follow-up assessments (Visits 3-6) |
In-person visit: Assessment Visits Measurement of height, weight, and completion of self-administered questionnaires via tablets. |
Remote and in-person visit: Assessment Visits Participants sent a secure survey link to complete self-administered questionnaires remotely Upon receipt of completed self-administered questionnaires, participants are scheduled for a brief (~15 minutes) in-person visit to collect anthropometric data. COVID-19 guidelines described above followed at all visits. |
2.2. Aims and hypotheses
The primary aim of SWOP is to test the causal effect of assignment to DSW on weight (primary outcome) and adoption of weight management practices (secondary outcome) over 24 months. We hypothesize that assignment to DSW will produce greater weight management (i.e., weight loss or stability) and greater initiation of weight management practices, including behaviors such as reducing calories, increasing exercise, and increasing dietary self-monitoring than SC. The secondary aim is to test the causal effect of adhering to DSW on weight and self-initiated adoption of weight management practices over 24 months. We hypothesize that adherence to DSW will produce greater weight management (i.e., weight loss or stability) and greater initiation of weight management practices. . The tertiary aim is to evaluate the cost-effectiveness of 24 months of DSW compared to SC from the perspective of patients, 3rd party payers and society. We hypothesize that DSW will be cost-effective compared to SC.
2.3. Participants and Setting
SWOP includes patients with obesity (BMI 30-50 kg/m2) receiving primary care services through a clinical network affiliated with an academic medical center. The clinical network is comprises six large primary care sites staffed by University-affiliated faculty and residents. Two sites are located on the University’s campus and adjoin the University Hospital, while four sites include satellite clinics located throughout the metropolitan area and surrounding communities.
Inclusion and Exclusion Criteria
Adults aged 19-65 years, with a BMI 30 – 50 kg/m2 and weighing ≤ 396 lbs (180 kg) will be recruited for this trial. The upper weight limit is due to the weight capacity of the Wifi-enabled scales used in this study. Individuals will be eligible if they meet the following inclusion criteria: 1) receives care at one of the participating primary care clinics; 2) resides within a 50-mile radius of the University’s main campus; 3) resides in one location at least 5 days each week; 4) possesses a smartphone and access to email to receive graphical weight feedback; and 5) has Wi-Fi Internet connection at home. Individuals will be excluded if they: 1) are pregnant or anticipating pregnancy; 2) are unwilling or unable to give informed consent, read English at the 5th-grade level, or accept random assignment; 3) are likely to no longer receive care from the primary care practices in the clinical network in the next 2 years; 4) have lost >10 lbs body weight in past 6 months (other than postpartum); 5) have had bariatric surgery in the past two years; 6) live with another household member already participating in this study; 7) used prescription weight loss medications within the past 6 months; and 8) are currently enrolled in a weight loss program.
2.4. Recruitment
Recruitment will include mailing postcards to patients identified through the review of the electronic medical record. Medical record information for patients in the University affiliated clinical network will be accessed through the institution’s Informatics for Integrating Biology and the Bedside framework, an established informatics network designed to support translational research by facilitating cohort identification based on inclusion and exclusion variables for study recruitment [32]. Using this resource, we identified approximately 25,000 unique patients meeting eligibility criteria (i.e., BMI 30-50 kg/m2; aged 19-65 years) who attended one of the primary care clinics in the affiliated network between January 2019 and March 2020. To obtain a balanced recruitment, the list was stratified by sex and race (white, non-white) and randomly recruited within strata. Our initial recruitment list is composed of 62% women and 38% men and includes 50% Whites, 45% African Americans, and 5% other racial/ethnic minorities. Additional recruitment strategies may include placing flyers within clinics (e.g., waiting room, exam rooms), word-of-mouth, clinicaltrials.gov, advertisements in University-affiliated publications, and clinical trial recruitment webpages. If more intensive recruitment strategies are needed, subsequent efforts will include more frequent visits to primary care practices to meet with staff to identify potential participants and direct recruitment of patients from waiting rooms.
2.5. Screening
Prospective participants will be initially pre-screened by a trained interviewer via telephone or online to determine initial eligibility and assess participant interest. Those initially eligible will be mailed (or electronically distributed) written materials describing the study and a copy of the consent form to review beforehand and then scheduled for an in-person screening and orientation visit. This in-person visit involves obtaining informed consent and contact information as well as confirming study eligibility (i.e., objective measures of weight and height to determine BMI). Eligible individuals will be scheduled for an in-person baseline and randomization visit.
2.6. Baseline and Randomization
At baseline (month 0), participants will have their height and weights measured and complete self-administered questionnaires (see Table 2). Participants will then be randomized, using stratified randomization to maintain the balance between groups within sex and race. Enrollment logs, one for each stratum, are prepared with a numerical sequence of identifiers and within each stratum a permuted block randomization is implemented. DSW and SC assignment will be randomly permuted within blocks of 4, 8, and 10. The blocks themselves will be randomly permuted. Each upcoming assignment will thus be unpredictable to prevent inadvertent bias.
Table 2.
Data collection /Assessments schedule
| Variable | Timepoint (Months) | ||||
|---|---|---|---|---|---|
| 0 | 6 | 12 | 18 | 24 | |
| Primary and Secondary Outcomes | |||||
| Height | X | ||||
| Weight | X | X | X | X | X |
| Adoption of Weight Control Practices and Goals | X | X | X | X | X |
| Self-efficacy for healthy eating, physical activity and weight loss | X | X | X | ||
| Maladaptive responses to DSW | |||||
| Eating Disorder Diagnostic Screening | X | X | X | ||
| Body-Q© Body Image | X | X | X | ||
| Body-Q© Psychological Function | X | X | X | X | X |
| Body-Q© Physical Function | X | X | X | X | X |
| Patient Health Questionnaire-8 | X | X | X | ||
| Frequency of Self-weighing | Ongoing | ||||
| Health Care Utilization and costs | X | X | X | X | X |
| Other Measures | |||||
| Demographics | X | ||||
| Medical History and Health Behaviors | X | X | X | X | X |
| Social Support | X | X | X | X | X |
| Use of monetary gift (SC only) | X | ||||
| COVID-19 Diagnosis and Impact | X | ||||
DSW, Daily self-weighing; SC, standard care
2.7. Treatment conditions
An overview of participant experiences is provided in Figure 2. During the baseline and randomization visit all enrolled participants receive tote bags and brief, evidence-based publicly-available educational materials (i.e., National Institute of Diabetes and Digestive and Kidney Diseases Healthy Eating & Physical Activity Across Your Lifespan Series from the Weight-control Information Network) [33]. These educational materials focus on dietary modification (e.g. increase fruits and vegetable intake), increasing physical activity (e.g., strategies for aerobic and muscle strengthening exercise), and behavioral strategies (e.g., self-monitoring, social support, stress management) to promote these lifestyle changes [33]. All participants will continue to receive routine care from their primary care provider. Because of numerous barriers and clinical demands [17–19], the intervention does not include direct involvement from primary care providers.
Figure 2.

Overview of participant experiences. DSW, daily self-weighing; SC, standard care
2.7.1. DSW Intervention
Participants randomized to DSW will receive a commercially-available electronic scale, the Withings Wi-Fi-enabled Body scale. This scale includes a weight trend screen displaying a line graph of recent weight recordings, automatic Wi-Fi synchronization, and is paired with a mobile application (Health Mate app, Withings SA, France) allowing the participants to access all weight data via their smartphone or other electronic devices. Research staff assists participants with setting up a Withings account and installing the Health Mate app on their mobile device. Participants’ Withings account login and password information will be collected to allow the research team to access scale data remotely throughout the study period. During the scale setup, participants are asked to weigh every day at the same time in light clothing (e.g., each morning immediately after voiding), view their weight and weight trajectory either on the scale’s digital display or via the Health Mate app, and instructed to be the only person using the scale until study completion. Although we cannot control scale usage by other household members, the Withings scale can recognize and differentiate between multiple users (i.e., multiple profiles can be setup). If participants choose to create other profiles, the scale will prompt the user to select the appropriate profile after weighing. When a weight value is not recognized as belonging to a study participant or other profile (i.e., if the weight value is a ±5% difference in body weight from the previous measurement), that value is not recorded or stored under the participants’ profile. These “unknown measurements” are stored separately on the app. Because of logistical, legal and regulatory barriers, participants’ weight measurements do not go into the electronic health record. However, participants are taught how to view and download their weight data from the Withings app and are encouraged to share their information with their primary care provider. To promote adherence to daily weighing, commercially-available and study-generated reminders and alerts are implemented. During scale setup, research staff assists participants with activating commercially-available electronic reminders imbedded within the Health Mate App, including daily phone reminders to weigh and weekly weight reports via email from Withings. Participants receive 3 study-generated alerts in the form of automated emails including 1) biweekly graphical feedback of weight plotted over a two-week period and containing a horizontal reference line indicating their starting weight, 2) reminders to weigh following brief periods of scale non-use (i.e., 3- and 7-days), and 3) alerts for > ±5% changes in body weight). To populate these automated emails, an application programming interface, a software intermediary that allows apps to “talk” to each other, was developed to retrieve and compile weight data. Study administrators also receive alerts for > ±5% changes in body weight and when participants do not weigh for 14 consecutive days. The study administrator or other staff will follow-up with participants following receipt of the 14-day non-use alert.
2.7.2. Standard Care
In addition to the standardized educational materials and routine care from their primary care provider, those randomized to SC will receive a monetary gift card containing $60 (the equivalent value to the scale) at the baseline visit. The purpose of giving the cash equivalent is to avoid the receipt of a valued item (the scale) being confounded with treatment assignment. SC participants will be provided a list of ideas about how they could use this monetary gift to support their weight management goals.
2.8. Treatment Fidelity
Scripts related to elements of the trial (e.g., study description, explanation of randomization and the two study conditions, receipt of the same educational materials) will be drafted and used by trained personnel. To measure fidelity, staff will also complete a brief checklist to ensure that they have adhered to elements of the protocol after each contact with participants.
2.9. Schedule of Data Collection and Assessments
Trained staff, blinded to random assignment, will serve as data collectors for assessment visits. The data collection schedule and study assessments are summarized in Table 2. Body weight (primary outcome), weight control practices (secondary outcome), and healthcare utilization and costs are measured at baseline, months 6, 12, 18, and 24. Self-administered questionnaires will be completed electronically via a REDCap survey link on iPads during in-person assessment visits. If needed, paper forms are available and will be double-entered into the database for quality control.
2.9.1. Anthropometry
Weight (primary outcome) is measured using a calibrated, electronic scale (Tanita WB-800S), to the nearest 0.1 kg. Height is measured using a wall-mounted stadiometer to the nearest 0.5 cm. Both measures will be performed without shoes. Weight and height are used to calculate the BMI.
2.9.2. Adoption of Weight Control Practices and Goals
A modified version of the 23-item Weight Control Practices Survey, previously used in the Look AHEAD trial [34–36], assesses a variety of weight control strategies, including weight loss attempts and participation in structured programs and/or self-directed weight control behaviors (e.g., reducing fat intake, maintaining food records, increasing physical activity) (secondary outcome). Participants indicate whether they had engaged in any of the behaviors and the duration of each behavior since their previous assessment [34–36]. Additional questions related to participants’ weight management goals (e.g., desire to lose, gain, or maintain weight), other behaviors (e.g., sedentary behaviors), and use of other weight management resources (e.g., websites, apps, and other electronic resources; health professionals) are included.
2.9.3. Frequency of self-weighing
Objective and subjective measure of the frequency of self-weighing are collected. The Withings scale records and automatically transmits scale usage data, in real time, providing objective information on participants’ adherence to the DSW protocol. The research team will access these data from participants’ Withings account. The frequency of objective self-weighing (number of weight measurements per week) will be calculated as the total number of measurements divided by the number of weeks of participation [30]. Self-reported measures of the frequency of self-weighing will be collected from all participants using the 23-item Weight Control Practices Survey (described in the next section) [34–36].
2.9.4. Self-Efficacy
Self-efficacy for healthy eating, physical activity and weight loss will be assessed using the 12-item Self-Efficacy for Weight Loss Trials Scale [37]. This measure has a 3-factor latent variable structure (physical activity, healthy eating, and weight loss) and predictive validity for weight-related outcomes (i.e., physical activity, fat intake, and weight).
2.9.5. Maladaptive Responses to DSW
Research consistently demonstrates that regular self-weighing does not induce or exacerbate maladaptive eating behaviors, body image dissatisfaction, or mood disturbance [26,27,38,39]. Nonetheless, to thoroughly monitor potential adverse effects of DSW, participants complete the Eating Disorder Diagnostic Screening (22-items) [40,41]; three subscales of the Body-Q© survey, including body image (7 items), psychological function (10 items), and physical function (7 items) [42,43]; and the Patient Health Questionnaire-8 [44,45]. These measures will be used to monitor symptoms of eating disorders, body image, and quality of life, and depressive symptoms, respectively. Any participants endorsing elevated scores on these measures will be referred to a member of the research team (clinical psychologist) qualified to conduct further assessment and offer referral for services as indicated.
2.9.6. Healthcare utilization and costs
Self-reported use of health care services (hospitalizations, ER/physician office visits, prescription drugs, etc.), out-of-pocket copayment costs and other expenses such as travel, and time spent in medical encounters, will be collected using survey questions currently used in our other primary care weight loss trial [46] and previously developed for the Look AHEAD and Encourage trials [47]. This survey also includes questions about other costs that may be affected by the intervention, such as expenses for gym memberships, exercise classes, meal replacement shakes or bars, or other programs to support weight loss efforts. We will also use the EuroQOL (EQ-5D-5L) to measure Quality Adjusted Life Years (QALYs) for cost-effectiveness analyses [48]. QALYs are a measure of health that combines length and quality of life. Years of life are combined with utility weights to adjust for quality of life. Weights have a value from 0 for death to 1 for perfect health, and one QALY corresponds to one year in perfect health.
2.10. COVID-19 modifications and COVID Diagnosis and Impact Survey
Aspects of the study protocol were modified due to COVID-19, including reducing in-person interactions and shifting to remote interactions, adopting HIPAA-compliant strategies to support a remote protocol (e.g., obtaining consent, completing self-administered forms electronically), and including retention-minded strategies to build rapport (Table 1). Because COVID-19 may impact health behaviors that, in turn, could influence the outcomes of this study, additional questions about whether participants experienced a COVID-19 diagnosis and the impact of COVID-19 on employment, food security, well-being, and dietary changes [49] were added to the protocol. Consistent with the participating University’s COVID-19 guidelines, 24 hours prior to in-person visits participants will be screened for COVID-19 and, if negative, are emailed instructions for a brief in-person visit. During in-person visits, staff and participants follow standard COVID-19 guidelines, including wearing personal protective equipment (e.g., masks), completing temperature scans, and screening questions prior to visit commencement. Study personnel regularly wash and/or disinfect hands and disinfect surfaces prior to and after use.
2.11. Retention
Retention procedures include the following: 1) providing monetary compensation ($50) and non-monetary incentives (e.g., phone accessories, towels, water bottles, keychains) at the end of each follow-up assessment (i.e., months 6, 12, 18, and 24), with an additional bonus cash incentive ($50) given at the end of the study for participants completing all assessments; 2) providing electronic newsletters (i.e., months 3, 9, 15, and 21) to serve as an intermediate communication point with participants between assessment visits. These newsletters express gratitude for continued participation, direct participants to publicly-available nutrition, physical activity, and wellness resources to support weight management, and highlight research staff to promote familiarity and affiliation with the study; 3) sending birthday and holiday cards during the study to further support engagement and retention; and 4) collecting details from participants for an additional contact, in the event that the participant cannot be contacted. We will also distinguish between those who are lost to follow-up or have decided to stop participating and those who do not comply but remain in the study. To retain the inferential strength of randomization, we will follow the intent-to-treat (ITT) principle, continuing to collect outcome data even if a participant does not adhere to DSW but is willing to have measurements taken. Such an approach has been advocated over imputing missing data [50].
2.12. Sample size and power
To test the causal effect of assignment to DSW (Aim 1), based on an ITT analysis, we consider the sample and effect sizes setting type I error rate of 0.05 (2-tailed) and 80% power. Because the primary outcome is weight change from baseline to 24 months, we conservatively estimate power for testing the difference between groups with a two-sample t-test, but in reality, we will use a more powerful analysis of covariance (ANCOVA) approach [51,52]. We assumed a standard deviation of weight change over a 2-year period of 7.8 kg; this value was chosen based on the reported values from similar studies [53–55]. Assuming those values, with 20% attrition, a total sample size of 400 (200 per group) would provide sufficient power to detect a mean difference of 2.2 kg in the 2-year weight change between groups, corresponding to a standardized mean difference of 0.28. With regard to testing the effect of adhering to DSW (Aim 2) we calculated per-protocol sample and effect size estimates. Conservatively assuming a DSW adherence rate of 65%, based on previous data from our group indicating DSW adherence >75% [30], a sample size of 200 per group would also give us 80% power to detect a between-groups mean difference of 2.4 kg at 2-years, corresponding to a standardized mean difference of 0.31. In the event of a 20% attrition rate, we would still have 80% power to detect a mean difference of 2.7 kg at 2 years. STATA 13.1 was used for these calculations.
2.13. Data Analytic Plan
We will use descriptive analyses to characterize the population and compare assignment groups. In the primary analyses, we will focus on the change in weight and adoption of weight control practices from baseline to follow-up (with baseline values as covariates) [51]. In general, the primary analysis will be conducted under the ITT principle at a 2-tailed alpha level of 0.05, with missing data handled using multiple imputation [56,57]. We will test assumptions of the parametric statistical method (i.e., normality and homoscedasticity of residuals) and, if violated, normalizing transformations (e.g., the Box-Cox transformation) and/or resampling-based nonparametric testing (e.g., permutation tests) will be used. The number of significance tests conducted in the primary analysis is modest and specified a priori. Hence, we will use no formal multiple testing correction. Instead, we will report exact p-values, allowing readers to know the raw uncorrected result and make their own judgments about statistical significance [56,58].
Specific Aim 1.
To examine the causal effect of DSW assignment on weight and adoption of weight management practices over 24 months, we will use an ANCOVA where the weight at the 24th month is regressed on the treatment indicator variable (coded 0/1) with adjustment for baseline weight [58]. The result is a difference-in-difference estimator for the treatment effect. Additional baseline covariates in the ANCOVA will include race, sex, and age. Secondary analyses will include all interim weight values using linear mixed models to account for the correlated structure of the repeated outcomes measurements. Mixed models will also allow us to handle the missingness in primary outcomes under the assumptions of missing at random [59]. We will use a multiple imputation technique with “auxiliary variables” to consider the uncertainty due to the missing data under the assumptions of not missing at random[57].
Specific Aim 2.
We will use a principal stratification approach to broken randomization experiments to determine the compiler average causal effect, which is the effect of the intervention in participants who would comply with their treatment assignment (i.e., protocol adherence) regardless of the randomized assignment. We will use a parametric pattern mixture model (i.e. censored normal model), which can handle both non-adherence and missingness in the primary outcome. In one analysis, we will calculate adherence as a continuous variable (number of days self-weighing/the total days in study period) and then stratify participants into two groups based on a median split (low adherence vs high adherence). The average causal effect will be examined in those with high adherence. An additional sensitivity analysis will include all interim weight values using the principal stratification approach. A growth mixture modelling framework will be used to identify the latent trajectories of outcomes of interest [60,61], with treatment-trajectory interactions tested using the likelihood ratio test. The adherence score will be defined cumulatively at each time point of follow-up in the sensitivity analysis.
2.14. Cost-Effectiveness Analysis (CEA)
Specific Aim 3.
The CEA will compare costs and outcomes of the two trial conditions. We will assess the cost-effectiveness of DSW compared to SC with a within-trial cost utility analysis from the perspectives of participants, health care sector, and society [62,63]. We will obtain implementation costs (e.g., cost of scales, research staff time) and participant’ cost which include medical costs (e.g., physician office visits, prescription drugs) and other costs (e.g., gym memberships, exercise equipment, weight loss products). We will also calculate the: 1) net cost of DSW; e.g., total cost of DSW (intervention implementation plus participants’ costs) minus total cost of SC (participants’ costs), and 2) effectiveness (e.g., the difference in QALYs between trial arms). QALYs will be calculated over the follow-up period using utility weights (EuroQOL scores) at each assessment point [64]. If the net cost of DSW is negative (DSW less costly) and DSW is more effective than SC, it will be considered cost-saving. If DSW has a positive net cost (DSW more costly) and it is more effective than SC, we will calculate an Incremental Cost Effectiveness Ratio (ICER), i.e., the ratio of net cost and QALYs gained, which represents how much it costs to achieve one additional unit of health benefit with the DSW instead of SC. DSW will be deemed cost effective if the ICER is below commonly used thresholds to determine cost-effectiveness [65]. Sensitivity analysis will be used to determine the robustness of results to the values used in the analysis (e.g., cost of scales, value of staff time, unit cost of physician visits).
3. Discussion
The SWOP trial will be the first randomized-controlled trial to rigorously test the effects of DSW among primary care patients with obesity as a simple, economically feasible and potentially scalable strategy to mitigate continued age-related weight gain. Most adults with obesity continue to gain weight and while primary care clinical guidelines recommend intensive weight management treatment for patients with obesity [14,66], significant barriers to delivery and patient uptake exist [17–19]. Given this, along with the challenges of losing weight and keeping it off, SWOP, a 24-month harm reduction intervention, focuses on disrupting continued age-related weight gain as a strategy to lessen the adverse health effects of obesity and its medical and economic consequences.
Adopting the harm reduction framework to the problem of age-related weight gain among adults with obesity holds promise in that: (1) continued weight gain among persons with obesity is common [5,6], (2) losing and sustaining a significant weight loss is difficult [67,68], and (3) fewer adults with obesity are attempting to lose weight [69]. Thus, promoting an increased awareness of weight status via DSW may serve as “stepladder” [70] to facilitate the initiation of incremental behavior changes conducive to weight management. Previous studies have typically included DSW as one of many strategies in a comprehensive multi-component intervention [71–73]; conversely, SWOP will isolate the effects of DSW as a potentially sustainable, “minimalist” strategy. Because DSW is a simple, low-cost strategy, it may be conducive for large-scale implementation within primary care, as well as a variety of other contexts (e.g., workplace programs, military). DSW can also be widely disseminated because of commercially-available, relatively low-cost scales.
In addition to examining the causal effect of assignment to DSW, this trial will evaluate whether there is a “dose-response” association between weighing frequency and weight management, which may have important implications for how to deliver, translate and scale DSW. SWOP also explores the costs and cost-effectiveness of DSW which has been neglected in prior research and is relevant to the broader implementation and dissemination of DSW within primary care. Further, results may inform future research in areas such as examining DSW’s “ripple effects” on patients’ family members; pairing DSW with telemedicine delivery and follow-up; investigating the effect of incentives to promote DSW adherence; and developing low-intensity interventions targeting other self-monitoring behaviors associated with weight management (e.g., dietary intake, screen time).
Interventions that seek to attenuate age-related weight gain that naturally occurs in the majority of US adults, particularly among those with obesity, are currently limited. Our DSW study attempts to address this gap by testing a potentially effective yet feasible approach for addressing obesity in the primary care setting. With plans to recruit a large sample with high levels of racial minority representation, we will increase the generalizability of our findings and may gain a better understanding of the impact of DSW among high-risk, underrepresented groups and related racial/ethnic differences in treatment. This trial includes 2 years of follow-up, which will provide important information about the initial and longer-term effects of DSW. Finally, SWOP is a pragmatic, remotely- delivered, low-intensity, and flexible intervention that supports sustainability and dissemination; this is even more salient in the context of COVID-19, which required only minimal changes to our original protocol.
Conclusions
Age-related weight gain among individuals with obesity is a major public health problem that imposes significant health, economic, and quality of life burdens. As the rate of obesity continues to increase among US adults, we may expect age-related weight gain to continue to also accelerate in the years to come. As such, developing ways to interrupt further weight gain is likely to confer significant benefits. SWOP is designed to evaluate whether DSW offers a simple way to curtail age-related weight gain. Successful completion of this trial should help us to answer the question whether DSW is a viable and cost-effective approach to mitigate continued weight gain among primary care patients.
Highlights.
Gradual weight gain during adulthood is common and may be pronounced among those with obesity.
Daily self-weighing (DSW) improves weight management in several populations and settings.
Effective yet practical strategies are needed for weight management in primary care.
DSW has not been tested as a weight management strategy in primary care.
Acknowledgments
Use of the Body-Q©, authored by Drs. Klassen, Pusic and Cano, was made under license from Memorial Sloan Kettering Cancer Center, New York, USA.
Funding
This work was supported by the National Institutes of Health [R01DK118939].
Abbreviations
- US
United States
- BMI
body mass index
- DSW
Daily self-weighing
- SWOP
Self-Weighing for Obesity Management in Primary Care
- SC
Standard care
- QALYs
Quality Adjusted Life Years
- ITT
intent-to-treat
- ANCOVA
analysis of covariance
- CEA
Cost-Effectiveness Analysis
- ICER
Incremental Cost Effectiveness Ratio
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Aff (Millwood). 2009; doi: 10.1377/hlthaff.28.5.w822 [DOI] [PubMed] [Google Scholar]
- [2].Cawley J, Meyerhoefer C. The medical care costs of obesity: an instrumental variables approach. J Health Econ. 2012; 10.1016/j.jhealeco.2011.10.003 [DOI] [PubMed] [Google Scholar]
- [3].Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity among adults and youth: United States, 2015–2016. NCHS data brief, no 288. Hyattsville, MD: National Center for Health Statistics. 2017. https://www.cdc.gov/nchs/data/databriefs/db288.pdfAccessed 15 April 2020 [Google Scholar]
- [4].Malhotra R, Ostbye T, Riley CM, Finkelstein EA. Young adult weight trajectories through midlife by body mass category. Obesity (Silver Spring). 2013; 10.1002/oby.20318 [DOI] [PubMed] [Google Scholar]
- [5].Finkelstein EA, Østbye T, Malhotra R. Body mass trajectories through midlife among adults with class I obesity. Surg Obes Relat Dis. 2013; 10.1016/j.soard.2012.01.004 [DOI] [PubMed] [Google Scholar]
- [6].GBD 2015 Obesity Collaborators, Afshin A, Forouzanfar MH, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017; 10.1056/NEJMoa1614362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Zheng Y, Manson J, Yuan C, Liang MH, et al. Associations of weight gain from early to middle adulthood with major health outcomes later in life. JAMA. 2017; 10.1001/jama.2017.7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Butryn ML, Phelan S, Hill JO, Wing RR. Consistent self-monitoring of weight: a key component of successful weight loss maintenance. Obesity (Silver Spring). 2007; 10.1038/oby.2007.368 [DOI] [PubMed] [Google Scholar]
- [9].Vetter ML, Faulconbridge LF, Webb VL, Wadden TA. Behavioral and pharmacologic therapies for obesity. Nat Rev Endocrinol. 2010; 10.1038/nrendo.2010.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Linde JA, Jeffery RW, French SA, Pronk NP, Boyle RG. Self-weighing in weight gain prevention and weight loss trials. Ann Behav Med. 2005; 10.1207/s15324796abm3003_5 [DOI] [PubMed] [Google Scholar]
- [11].Pacanowski CR, Levitsky DA. Frequent self-weighing and visual feedback for weight loss in overweight adults. J Obes. 2015; 10.1155/2015/763680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Madigan CD, Jolly K, Lewis AL, Aveyard P, Daley AJ. A randomised controlled trial of the effectiveness of self-weighing as a weight loss intervention. Int J Behav Nutr Phys Act. 2014; 10.1186/s12966-014-0125-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].VanWormer JJ, Martinez AM, Martinson BC, Crain AL, Benson GA, Cosentino DL, Pronk NP. Self-weighing promotes weight loss for obese adults. Am J Prev Med. 2009; 10.1016/j.amepre.2008.09.022 [DOI] [PubMed] [Google Scholar]
- [14].Jensen MD, Ryan DH, Apovian CM et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014; 10.1161/01.cir.0000437739.71477.ee [DOI] [PubMed] [Google Scholar]
- [15].Moyer VA, U.S. Preventive Services Task Force. Screening for and management of obesity in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012; 10.7326/0003-4819-157-5-201209040-00475 [DOI] [PubMed] [Google Scholar]
- [16].Tsai AG, Remmert JE, Butryn ML, Wadden TA. Treatment of obesity in primary care. Med Clin North Am. 2018; 10.1016/j.mcna.2017.08.005 [DOI] [PubMed] [Google Scholar]
- [17].Foster GD, Wadden TA, Makris AP, Davidson D, Sanderson RS, Allison DB, Kessler A. Primary care physicians’ attitudes about obesity and its treatment. Obes. Res. 2003; 10.1038/oby.2003.161 [DOI] [PubMed] [Google Scholar]
- [18].Kushner RF. Barriers to providing nutrition counseling by physicians: a survey of primary care practitioners . Prev Med. 1995; 10.1006/pmed.1995.1087 [DOI] [PubMed] [Google Scholar]
- [19].Ruelaz AR, Diefenbach P, Simon B, Lanto A, Arterburn D, Shekelle PG. Perceived barriers to weight management in primary care--perspectives of patients and providers. J Gen Intern Med. 2007; 10.1007/s11606-007-0125-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Tsai AG, Wadden TA. Treatment of Obesity in Primary Care Practice in the United States: A Systematic Review. J Gen Intern Med. 2009; 10.1007/s11606-009-1042-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Martin PD, Dutton GR, Rhode PC, Horswell RL, Ryan DH, Brantley PJ. Weight loss maintenance following a primary care intervention for low-income minority women. Obesity (Silver Spring). 2008; 10.1038/oby.2008.399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Tsai AG, Wadden TA, Rogers MA, Day SC, Moore RH, Islam BJ. A primary care intervention for weight loss: results of a randomized controlled pilot study. Obesity (Silver Spring). 2010; 10.1038/oby.2009.457 [DOI] [PubMed] [Google Scholar]
- [23].Kumanyika SK, Fassbender JE, Sarwer DB, et al. One-year results of the Think Health! study of weight management in primary care practices. Obesity (Silver Spring). 2012; 10.1038/oby.2011.329 [DOI] [PubMed] [Google Scholar]
- [24].Hawk M, Coulter RWS, Egan JE, et al. Harm reduction principles for healthcare settings, Harm Reduct J 2017; 10.1186/s12954-017-0196-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Zheng Y, Klem ML, Sereika SM, Danford CA, Ewing LJ, Burke LE. Self-weighing in weight management: a systematic literature review. Obesity (Silver Spring). 2015; 10.1002/oby.20946 [DOI] [PubMed] [Google Scholar]
- [26].Pacanowski CR, Bertz F, Levitsky DA. Daily self-weighing to control body weight in adults: A critical review of the literature. SAGE Open. 2014; 10.1177/2158244014556992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Gokee-LaRose J, Gorin AA, Wing RR. Behavioral self-regulation for weight loss in young adults: a randomized controlled trial. Int J Behav Nutr Phys Act. 2009; 10.1186/1479-5868-6-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Levitsky DA, Garay J, Nausbaum M, Neighbors L, Dellavalle DM. Monitoring weight daily blocks the freshman weight gain: a model for combating the epidemic of obesity. Int J Obes (Lond). 2006; 10.1038/sj.ijo.0803221 [DOI] [PubMed] [Google Scholar]
- [29].Wing RR, Tate DF, Espeland MA et al. Innovative self-regulation strategies to reduce weight gain in young adults: the study of novel approaches to weight gain prevention (SNAP) randomized clinical trial. JAMA Intern Med. 2016; 10.1001/jamainternmed.2016.1236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bertz F, Pacanowski CR, Levitsky DA, Frequent self-weighing with electronic graphic feedback to prevent age-related weight gain in young adults. Obesity (Silver Spring). 2015; 10.1002/oby.21211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Wilkinson L, Pacanowski CR, Levitsky D. Three-year follow-up of participants from a self-weighing randomized controlled trial. J Obes. 2017; 10.1155/2017/4956326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Informatics for Integrating Biology and the Bedside (i2B2). https://www.i2b2.org/.Accessed 8 February 2021
- [33].US Department of Health and Human Services, National Institutes of Health, WIN Weight Control Network. Better Health and You: Tips for Adults. NIH Publication No. 08-4992, 2012. Available at: https://www.niddk.nih.gov/-/media/Files/Weight-Management/tipsforadults804bw.pdfAccessed 16 March 2021
- [34].Neumark-Sztainer D, Sherwood NE, French SA, Jeffery RW. Weight control behaviors among adult men and women: cause for concern? Obes Res. 1999; 10.1002/j.1550-8528.1999.tb00700.x [DOI] [PubMed] [Google Scholar]
- [35].Raynor HA, Jeffery RW, Ruggiero AM, Clark JM, Delahanty LM. Weight loss strategies associated with BMI in overweight adults with type 2 diabetes at entry into the Look AHEAD (Action for Health in Diabetes) trial. Diabetes Care. 2008; 10.2337/dc07-2295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Look AHEAD Research Group. Eight-year weight losses with an intensive lifestyle intervention: the look AHEAD study. Obesity (Silver Spring). 2014; 10.1002/oby.20662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Wilson KE, Harden SM, Almeida FA et al. Brief self-efficacy scales for use in weight-loss trials: preliminary evidence of validity. Psychol. Assess. 2016; 10.1037/pas0000249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Steinberg DM, Tate DF, Bennett GG, Ennett S, Samuel-Hodge C, Ward DS. Daily self-weighing and adverse psychological outcomes: a randomized controlled trial. Am J Prev Med. 2014; 10.1016/j.amepre.2013.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Welsh EM, Sherwood NE, VanWormer JJ, Hotop AM, Jeffery RW. Is frequent self-weighing associated with poorer body satisfaction? Findings from a phone-based weight loss trial. J Nutr Educ Behav. 2009; 10.1016/j.jneb.2009.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Stice E, Telch CF, Rizvi SL. Development and validation of the eating disorder diagnostic scale: a brief self-report measure of anorexia, bulimia, and binge-eating disorder., Psychol Assess. 2000; 10.1037//1040-3590.12.2.123 [DOI] [PubMed] [Google Scholar]
- [41].Stice E, Fisher M, Martinez E. Eating disorder diagnostic scale: additional evidence of reliability and validity. Psychol Assess. 2004; 10.1037/1040-3590.16.1.60. [DOI] [PubMed] [Google Scholar]
- [42].Klassen AF, Cano SJ, Scott A, Tsangaris E, Pusic AL. Assessing outcomes in body contouring. Clin Plast Surg. 2014; 10.1016/j.cps.2014.06.004 [DOI] [PubMed] [Google Scholar]
- [43].Klassen AF, Cano SJ, Alderman A, et al. The BODY-Q: a patient-reported outcome instrument for weight loss and body contouring treatments. Plast Reconstr Surgery Glob Open. 2016; 10.1097/GOX.0000000000000665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kroenke K, Strine TW, Spitzer RL, Williams JBW, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. 2009; 10.1016/j.jad.2008.06.026 [DOI] [PubMed] [Google Scholar]
- [45].Razykov I, Ziegelstein RC, Whooley MA, Thombs BD. The PHQ-9 versus the PHQ-8--is item 9 useful for assessing suicide risk in coronary artery disease patients? Data from the heart and soul study. J Psychosom Res. 2012; 10.1016/j.jpsychores.2012.06.001 [DOI] [PubMed] [Google Scholar]
- [46].Dutton GR, Lewis CE, Cherrington A, et al. A weight loss intervention delivered by peer coaches in primary care: Rationale and study design of the PROMISE trial. Contemp Clin Trials. 2018; 10.1016/J.CCT.2018.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Espeland MA, Glick HA, Bertoni A, et al. Impact of an intensive lifestyle intervention on use and cost of medical services among overweight and obese adults with type 2 diabetes: the action for health in diabetes. Diabetes Care. 2014; 10.2337/dc14-0093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Dyer MTD, Goldsmith KA, Sharples LS, Buxton MJ. A review of health utilities using the EQ-5D in studies of cardiovascular disease. Health Qual Life Outcomes. 2010; 10.1186/1477-7525-8-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Pennington Biomedical COVID-19 Survey. Availible from: https://www.phenxtoolkit.org/toolkit_content/PDF/PBRC_Covid.pdf.Accessed 8 February 2021
- [50].Kaiser KA, Affuso O, Desmond R, Allison DB. Baseline participant characteristics and risk for dropout from ten obesity randomized controlled trials: a pooled analysis of individual level data. Front Nutr. 2014; 10.3389/fnut.2014.00025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Allison DB. When is it worth measuring a covariate in a randomized clinical trial? J Consult Clin Psychol. 1995; 10.1037//0022-006x.63.3.339 [DOI] [PubMed] [Google Scholar]
- [52].Allison DB, Allison RL, Faith MS, Paultre F, Pi-Sunyer FX. Power and money: Designing statistically powerful studies while minimizing financial costs. Psychol Methods. 1997; 10.1037/1082-989X.2.1.20 [DOI] [Google Scholar]
- [53].Sherwood NE, Crain AL, Martinson BC, et al. Enhancing long-term weight loss maintenance: 2 year results from the Keep It Off randomized controlled trial. Prev Med (Baltim). 2013; 10.1016/J.YPMED.2012.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Levitsky DA, Halbmaier CA, Mrdjenovic G. The freshman weight gain: a model for the study of the epidemic of obesity. Int J Obes Relat Metab Disord. 2004; 10.1038/sj.ijo.0802776 [DOI] [PubMed] [Google Scholar]
- [55].French SA, Jeffery RW, Murray D. Is dieting good for you?: Prevalence, duration and associated weight and behaviour changes for specific weight loss strategies over four years in US adults. Int J Obes Relat Metab Disord. 1999; 10.1038/sj.ijo.0800822 [DOI] [PubMed] [Google Scholar]
- [56].Allison DB, Brown AW, George BJ, Kaiser KA. Reproducibility: A tragedy of errors. Nature. 2016; 10.1038/530027a [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Li P, Stuart EA, Allison DB. Multiple imputation: a flexible tool for handling missing data. JAMA. 2015; 10.1001/jama.2015.15281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Allison DB, Gorman B, Primavera L. The most common questions asked of statistical consultants: our favorite responses and recommended readings. Journal of Group Psychotherapy, Psychodrama & Sociometry. 1993 [Google Scholar]
- [59].Xun P, He Q. Multivariate Analysis. In: Handbook on Medical Statistics. Singapore: World Scientific Publishing Co pte Ltd; 2017. p. 103–144. [Google Scholar]
- [60].Muthén BO. Beyond SEM: General latent variable modeling. Behaviormetrika. 2002; 10.2333/bhmk.29.81 [DOI] [Google Scholar]
- [61].Muthén B Latent variable analysis: Growth mixture modeling and related techniques for longitudinal data, In: Handbook of quantitative methodology for the social sciences. Newbury Park: Sage; 2004. p. 345–368. [Google Scholar]
- [62].Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016; 10.1001/jama.2016.12195 [DOI] [PubMed] [Google Scholar]
- [63].Ramsey SD, Willke RJ, Glick H, et al. Cost-effectiveness analysis alongside clinical trials II-An ISPOR Good Research Practices Task Force report. Value Health. 2015; 10.1016/j.jval.2015.02.001 [DOI] [PubMed] [Google Scholar]
- [64].Hunter RM, Baio G, Butt T, Morris S, Round J, Freemantle N. An educational review of the statistical issues in analysing utility data for cost-utility analysis. Pharmacoeconomics. 2015; 10.1007/s40273-014-0247-6 [DOI] [PubMed] [Google Scholar]
- [65].Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness--the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014; 10.1056/NEJMp1405158 [DOI] [PubMed] [Google Scholar]
- [66].McTigue KM, Harris R, Hemphill B, et al. Screening and interventions for obesity in adults: summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2003; 10.7326/0003-4819-139-11-200312020-00013 [DOI] [PubMed] [Google Scholar]
- [67].Dutton GD, Perri MG. Delivery, evaluation, and future directions for cognitive-behavioral treatments of obesity., In: The Oxford handbook of cognitive and behavioral therapies. New York: Oxford University Press; 2016. p. 419–437. [Google Scholar]
- [68].McGuire MT, Wing RR, Klem ML, Lang L, Hill JO. What predicts weight regain in a group of successful weight losers? J Consult Clin Psychol. 1999; 10.1037//0022-006x.67.2.177 [DOI] [PubMed] [Google Scholar]
- [69].Snook KR, Hansen AR, Duke CH, Finch KC, Hackney AA, Zhang J. Change in percentages of adults with overweight or obesity trying to lose weight, 1988-2014., JAMA. 2017 10.1001/jama.2016.20036 [DOI] [PubMed] [Google Scholar]
- [70].Young S Stick with It: A Scientifically Proven Process for Changing Your Life-for Good, Harper, New York, 2017. [Google Scholar]
- [71].Wing RR, Tate DF, Gorin AA, Raynor HA, Fava JL. A self-regulation program for maintenance of weight loss. N Engl J Med. 2006; 10.1056/NEJMoa061883 [DOI] [PubMed] [Google Scholar]
- [72].Funk KL, Stevens VJ, Appel LJ, et al. Associations of internet website use with weight change in a long-term weight loss maintenance program. J Med Internet Res. 2010; 10.2196/jmir.1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Wing RR, Crane MM, Thomas JG, Kumar R, Weinberg B. Improving weight loss outcomes of community interventions by incorporating behavioral strategies. Am J Public Health. 2010; 10.2105/AJPH.2009.183616 [DOI] [PMC free article] [PubMed] [Google Scholar]


