Abstract
Repetitive transcranial magnetic stimulation (rTMS) is a non‐invasive brain stimulation method increasingly used to treat psychiatric disorders, primarily depression. Initial studies suggest that rTMS may help to treat addictions, but evaluation in multicenter randomized controlled trials (RCTs) is needed. We conducted a multicenter double‐blind RCT in 262 chronic smokers meeting DSM‐5 criteria for tobacco use disorder, who had made at least one prior failed attempt to quit, with 68% having made at least three failed attempts. They received three weeks of daily bilateral active or sham rTMS to the lateral prefrontal and insular cortices, followed by once weekly rTMS for three weeks. Each rTMS session was administered following a cue‐induced craving procedure, and participants were monitored for a total of six weeks. Those in abstinence were monitored for additional 12 weeks. The primary outcome measure was the four‐week continuous quit rate (CQR) until Week 18 in the intent‐to‐treat efficacy set, as determined by daily smoking diaries and verified by urine cotinine measures. The trial was registered at ClinicalTrials.gov (NCT02126124). In the intent‐to‐treat analysis set (N=234), the CQR until Week 18 was 19.4% following active and 8.7% following sham rTMS (X2=5.655, p=0.017). Among completers (N=169), the CQR until Week 18 was 28.0% and 11.7%, respectively (X2=7.219, p=0.007). The reduction in cigarette consumption and craving was significantly greater in the active than the sham group as early as two weeks into treatment. This study establishes a safe treatment protocol that promotes smoking cessation by stimulating relevant brain circuits. It represents the first large multicenter RCT of brain stimulation in addiction medicine, and has led to the first clearance by the US Food and Drug Administration for rTMS as an aid in smoking cessation for adults.
Keywords: Smoking cessation, repetitive transcranial magnetic stimulation, cigarette consumption, cigarette craving, lateral prefrontal cortex, insula, addiction medicine
Transcranial magnetic stimulation (TMS) non‐invasively stimulates neuronal tissue in awake humans and has been used in research since 1985 and in clinical practice since 20081. Brief electric pulses are delivered using an electromagnetic coil placed over selected brain areas, which induce electrical currents in the underlying cortical tissue and neuronal depolarization2.
Repetitive TMS (rTMS) pulses applied in daily sessions can induce long‐term modification in mood and behavior1. Following multicenter randomized controlled trials (RCTs) that demonstrated both safety and efficacy, specific rTMS coils and protocols have been used in the treatment of depression and obsessive‐compulsive disorder3, 4, 5. In these conditions, rTMS can serve as an alternative for patients who cannot tolerate medication side effects, or who do not sufficiently benefit from pharmacological or psychotherapeutic options.
Substance use disorders affect hundreds of millions of people globally. Treatment options are limited, despite advances in neuroscience that have started to elucidate the brain regions involved6, 7. Tobacco use disorder is the most common substance use disorder in many countries worldwide. It is characterized by craving and withdrawal, compulsive use despite negative consequences, and repeated relapses, and is associated with multiple health problems and failed attempts at cessation8, 9, 10, 11.
Animal and small sample size human studies have demonstrated that rTMS of the prefrontal cortex affects the neural substrate of substance use disorders and reduces craving and consumption of substances of abuse, including nicotine12, 13, 14, 15, 16, 17, 18. The majority of studies applied focal rTMS over the dorsolateral prefrontal cortex, while a previous pilot study from our group targeted deeper layers of the lateral prefrontal and insular cortices of subjects with tobacco use disorder19, 20. In that study, 15 active rTMS sessions (20 min/weekday for three weeks), compared to sham, induced a significantly higher quit rate and reduced cigarette consumption. Increased inhibitory control over the compulsive desire to smoke and disruption of circuits associated with craving were proposed as mechanisms accounting for the therapeutic effect19.
Here, we report the results of a prospective multicenter double‐blind RCT, which was based on our pilot study and followed the recommendations of a consensus paper outlining the criteria for brain stimulation studies in substance use disorders21. This trial has led to the first clearance by the US Food and Drug Administration (FDA) for rTMS as an aid in smoking cessation for adults.
METHODS
Study design and participants
The study was conducted in the US (12 sites) and Israel (two sites), with active enrollment from August 2014 through August 2019. The trial protocol was approved by local institutional review boards and registered at clinicaltrials.gov (NCT02126124).
We included adults aged 22‐70 years who were chronic smokers (at least ten cigarettes/day for at least one year) and met the DSM‐5 criteria for tobacco use disorder8. In addition, participants had to be motivated to quit (replying “very likely” or “somewhat likely” to a motivation questionnaire) and with no period of abstinence of more than three months in the past year. All subjects provided informed consent for participation in the study, and gave satisfactory answers on a safety screening questionnaire for TMS22.
Key exclusion criteria were current treatment for smoking, use of nicotine other than through cigarettes, any other active psychiatric disorder diagnosed according to the DSM‐5, any other substance use disorder during the last 12 months before recruitment, use of any psychotropic medication on a regular basis, history of epilepsy or seizures (except those therapeutically induced by electroconvulsive therapy) or increased risk of seizures for any reason, any significant neurological disorder or insult, history of any metal in the head (outside the mouth) or metallic implant, and known or suspected pregnancy or lactation.
Procedures
Eligible participants were randomized and allocated to treatment groups (1:1). A central interactive web‐based randomization system assigned a unique participant randomization code, which matched pre‐programmed cards maintained at the centers and determined the nature of rTMS (active or sham), such that participants, operators and raters were blinded to the treatment condition.
The timeline for treatment and assessments is provided in Figure 1. Following randomization and selection of a target quit date within the first two weeks of treatment (“grace period”), daily rTMS (active or sham) was applied for three weeks (five sessions/week), while subjects provided daily smoking diaries and (once a week) urine samples for assessment of cotinine levels. At each visit, the number of cigarettes smoked was recorded through the Nicotine Use Inventory (NUI), and adverse events were monitored. The Tobacco Craving Questionnaire (TCQ)23 and the Fagerström Test for Nicotine Dependence (FTND)24 were administered weekly.
Figure 1.
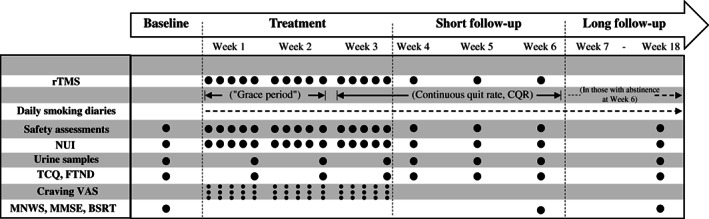
Timeline for treatment and assessments. rTMS – repetitive transcranial magnetic stimulation (active or sham), NUI – Nicotine Use Inventory, TCQ – Tobacco Craving Questionnaire, FTND – Fagerström Test for Nicotine Dependence, VAS – Visual Analogue Scale (before provocation procedure, after provocation procedure, and after rTMS session), MNWS – Minnesota Nicotine Withdrawal Scale, MMSE – Mini‐Mental State Examination, BSRT – Buschke Selective Reminding Test
An additional three weeks of once‐weekly rTMS were then delivered, while participants continued to provide daily smoking diaries. Urine samples were collected, adverse events monitored, and the TCQ and FTND administered at each visit. Participants who were abstinent at the last visit (Week 6) were invited for a long‐term follow‐up, with an additional visit four months after the “grace period” (Week 18). Abstinence was defined as a self‐report of no smoking (zero cigarettes/day) confirmed by urine cotinine levels lower than 200 ng/ml25, 26. During the long‐term follow‐up, subjects kept on providing daily smoking diaries. Urine samples were collected, adverse events monitored, and the TCQ and FTND administered at Week 18.
The Minnesota Nicotine Withdrawal Scale (MNWS)27 (both self‐reported and observer‐reported), the Mini‐Mental State Examination (MMSE)28 and the Buschke Selective Reminding Test (BSRT)29 were administered at baseline and at Weeks 6 and 18 to assess withdrawal symptoms and cognition.
Treatment was delivered using a Magstim Rapid2 TMS stimulator (Magstim, UK) equipped with the H4‐coil (BrainsWay, Israel). The H4 coil has been shown to bilaterally stimulate neuronal pathways in the lateral prefrontal cortex and insula with an intensity above the neuronal threshold for activation19, 30 (see supplementary information).
For each participant, the rTMS intensity was set using the individual’s minimal motor threshold, which was obtained by localizing the optimal helmet position on the scalp for activation of the right abductor pollicis brevis muscle19. The helmet was then aligned symmetrically and moved 6 cm anteriorly. Each participant was assigned a unique magnetic card that, when inserted into the TMS machine, determined which coil within the helmet (active or sham) would be used. The sham coil (encased in the same helmet) induced acoustic and scalp sensations similar to those induced by the active coil, but without electromagnetic penetration into the brain and without neural activation4, 19. The intensity of the stimulator was set to 120% of the minimal motor threshold. Sixty rTMS trains of 30 pulses (i.e., a total of 1,800 pulses) were applied at 10 Hz (3 sec each train) with 15 sec inter‐train intervals.
Participants were instructed to refrain from smoking for at least two hours prior to each visit. Each rTMS session was preceded by a 5‐min provocation procedure, which included participants imagining their greatest trigger for craving, listening to an audio script with instructions to handle a cigarette and a lighter, and viewing pictures of smoking (see supplementary information). Craving was assessed three times: before the provocation procedure, after the provocation, and after the rTMS session (Visual Analogue Scale, VAS – respectively, VAS1, VAS2 and VAS3). Following each rTMS session, a short (~2 min) motivational talk based on the booklet “Clearing the Air”, and supporting the decision to quit, was read to each participant31 (see supplementary information).
Outcome measures
The primary outcome measure was the four‐week continuous quit rate (CQR) until Week 18 among participants composing the intent‐to‐treat efficacy set (i.e., the percentage of quitters among all randomized participants who met eligibility criteria and had at least one post‐baseline assessment). Secondary endpoints included the CQR until Week 18 in the completer analysis set, the CQR until Week 6, and changes in cigarette consumption and craving.
Criteria for discontinuation included missing three consecutive sessions or four total sessions, or the occurrence of a serious adverse event.
Statistical analysis
The weighted average of our pilot study and former pharmacological studies resulted in a difference of about 20% in abstinence rates between the treatment and control groups19, 32, 33, 34, 35. Aiming at this difference between groups and a 80% power with a two‐sided level of significance of 5%, and allowing for a potential 40% drop‐out, a total of about 270 participants were required.
The CQR was compared between the study groups by a chi‐squared test and modeled with logistic regression. The number of cigarettes smoked and TCQ scores were presented over time and analyzed with a repeated measures analysis of covariance model. Craving VAS scores were presented over time and analyzed with a repeated measures analysis.
For comparison of means, the two‐sample t‐test or the Wilcoxon rank‐sum test was used. For comparison of proportions, the chi‐squared test or Fisher’s exact test was used, as appropriate. The hierarchical approach was adopted for the planned endpoints to control for type I error (i.e., analyzing the next endpoint in the hierarchy only if the previous endpoint analysis was found significant). Nominal p values are presented.
A detailed description of the statistical analysis is provided in the supplementary information.
RESULTS
Characteristics of the patients
A total of 262 participants were enrolled in the study, with 123 randomized to receive active rTMS and 139 sham rTMS. The intent‐to‐treat efficacy sample included the 234 randomized participants who had at least one post‐baseline assessment. The completer analysis sample included the 169 randomized participants who completed the three weeks of treatment and the measures relevant to the four‐week CQR determination (following the “grace period”) at Week 6. The CONSORT diagram is provided in the supplementary information.
No statistically significant differences were found between the study groups with respect to baseline demographic or clinical data, including nicotine withdrawal and craving assessment scales, except for the MNWS observer‐reported scores (see Table 1). Participants in the active group had been smoking for an average of 27.1±13.0 years, while those in the sham group for an average of 26.2±13.7 years. All participants had made at least one prior failed attempt to quit using various methods, with 68% having made at least three failed attempts, and 27% having made more than five failed attempts (see Table 1).
Table 1.
Demographic and clinical features of patients randomized to receive active or sham repetitive transcranial magnetic stimulation
| Active (N=123) | Sham (N=139) | p | |
|---|---|---|---|
| Gender (% female) | 48.8 | 47.5 | 0.834 |
| Age (years, mean±SD) | 45.0±13.0 | 44.8±13.4 | 0.946 |
| Years of education (%) | 45.0±13.0 | 44.8±13.4 | 0.946 |
| <9 | 0 | 1.4 | 0.074 |
| 9 to 12 | 33.3 | 23.0 | |
| >12 | 66.7 | 75.5 | |
| Marital status (%) | |||
| Married | 23.6 | 28.8 | 0.091 |
| Single | 54.5 | 39.6 | |
| Divorced | 17.1 | 26.6 | |
| Widowed | 4.9 | 5.0 | |
| Age started smoking (years, mean±SD) | 16.9±4.0 | 17.4±5.3 | 0.390 |
| Total years smoking (years, mean±SD) | 27.1±13.0 | 26.2±13.7 | 0.597 |
| N. cigarettes/day (mean±SD) | 18.3±7.7 | 18.2±7.2 | 0.874 |
| Desire to quit (from 1 ‐ low to 10 ‐ high, mean±SD) | 8.8±1.4 | 9.0±1.3 | 0.238 |
| N. tries to stop (%) | |||
| One | 14.3 | 21.9 | 0.283 |
| Two | 10.9 | 16.1 | |
| Three | 23.5 | 18.2 | |
| Four | 11.8 | 9.5 | |
| Five | 12.6 | 7.3 | |
| More than five | 26.9 | 27.0 | |
| Longest period without smoking (%) | |||
| 1 week or less | 26.7 | 26.1 | 0.728 |
| >1 week to 1 month | 19.2 | 13.8 | |
| >1 month to 6 months | 25.0 | 26.1 | |
| >6 months to 1 year | 12.5 | 12.3 | |
| Longer than 1 year | 16.7 | 21.7 | |
| Previous stopping methods | |||
| Bupropion | 12.4 | 10.1 | 0.566 |
| Varenicline | 24.0 | 25.4 | 0.795 |
| Nicotine patch | 33.9 | 35.5 | 0.784 |
| Nicotine gum | 27.3 | 26.8 | 0.934 |
| Nicotine lozenge | 9.1 | 10.1 | 0.774 |
| Nicotine oral inhaler | 5.8 | 4.3 | 0.597 |
| Cold turkey | 73.6 | 76.8 | 0.544 |
| CBT or other psychotherapy | 3.3 | 2.9 | 1.000 |
| Hypnosis | 10.7 | 5.8 | 0.146 |
| Other | 21.5 | 18.1 | 0.496 |
| Tobacco Craving Questionnaire total score (mean±SD) | 44.9±15.8 | 42.7±18.1 | 0.291 |
| Fagerström Test for Nicotine Dependence (mean±SD) | 5.5±2.0 | 5.3±2.0 | 0.268 |
| Minnesota Nicotine Withdrawal Scale, self‐reported (mean±SD) | 7.6±5.4 | 8.1±6.1 | 0.450 |
| Minnesota Nicotine Withdrawal Scale, observer‐reported (mean±SD) | 0.8±1.4 | 1.4±1.9 | 0.005 |
CBT – cognitive behavioral therapy
Efficacy analysis
The CQR was significantly higher in the active group until both Week 6 and Week 18 (Figure 2). The CQR of completers until Week 6 was 25.3% for the active group and 6.4% for the sham group (X2=11.885, p=0.0006). Only participants who were abstinent at the Week 6 visit were followed up to Week 18. Of these participants, 63% (active group) and 50% (sham group) remained non‐smokers until Week 18 (X2=8.46, p=0.003). In the intent‐to‐treat set, the CQR until Week 18 was 19.4% for the active group and 8.7% for the sham group (X2=5.655, p=0.017), while in completers it was 28.0% and 11.7%, respectively (X2=7.219, p=0.007).
Figure 2.
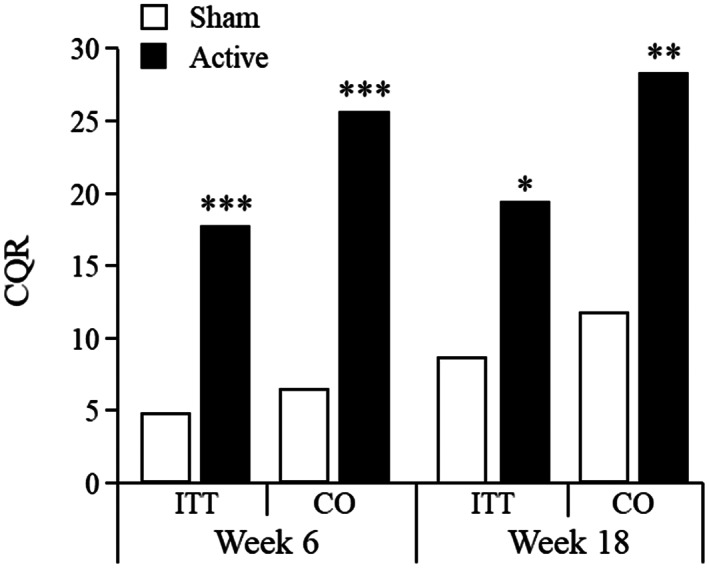
Four‐week continuous quit rate (CQR) until Week 6 and Week 18 in patients receiving active or sham repetitive transcranial magnetic stimulation. Only participants who were abstinent at Week 6 were followed up to Week 18. ITT – intent‐to‐treat set, CO – completer analysis set. *p<0.05, **p<0.01, ***p<0.001.
The number of cigarettes smoked and the TCQ total score (craving levels) decreased significantly more in the active than in the sham group at each week following the target quit date, in both the intent‐to‐treat and the completer analysis sets, with the only exception of the TCQ total score at Week 5 in the intent‐to‐treat set, for which statistical significance was only approached (see Table 2).
Table 2.
Differences (active minus sham) in number of cigarettes smoked and change from baseline in Tobacco Craving Questionnaire (TCQ) total score at each week of treatment
| Week | Number of cigarettes smoked | Change from baseline in TCQ total score | ||
|---|---|---|---|---|
| Adjusted mean difference (95% CI) | p | Adjusted mean difference (95% CI) | p | |
| Intent‐to‐treat set | ||||
| 2 | –16.64 (–27.91 to –5.37) | 0.004 | –3.94 (–8.63 to 0.76) | 0.100 |
| 3 | –19.14 (–31.14 to –7.14) | 0.002 | –7.17 (–12.16 to –2.18) | 0.005 |
| 4 | –18.02 (–30.22 to –5.82) | 0.004 | –6.44 (–11.52 to –1.35) | 0.013 |
| 5 | –18.87 (–31.27 to –6.48) | 0.003 | –4.83 (–9.99 to 0.33) | 0.067 |
| 6 | –16.14 (–28.79 to –3.48) | 0.012 | –5.56 (–10.70 to –0.42) | 0.034 |
| Completer analysis set | ||||
| 2 | –20.35 (–32.73 to –7.98) | 0.001 | –5.50 (–10.56 to –0.43) | 0.033 |
| 3 | –19.18 (–31.66 to –6.69) | 0.003 | –7.69 (–12.78 to –2.61) | 0.003 |
| 4 | –16.56 (–29.08 to –4.05) | 0.010 | –5.97 (–11.04 to –0.89) | 0.021 |
| 5 | –18.55 (–31.15 to –5.95) | 0.004 | –5.61 (–10.71 to –0.50) | 0.031 |
| 6 | –15.01 (–27.85 to –2.17) | 0.022 | –5.71 (–10.81 to –0.62) | 0.028 |
The average difference in total number of cigarettes smoked from baseline until Week 6 between the active and the sham groups was –79.9 (95% CI: –136.69 to –23.05, p=0.0061) in the intent‐to‐treat set and –95.5 (95% CI: –159.16 to –31.91, p=0.0035) in the completer analysis set. The average weekly reduction in cigarette consumption was significantly greater in the active group (adjusted mean weekly difference between groups = 15.01, 95% CI: 2.17‐27.85, p=0.022).
The average weekly reduction in TCQ total score was also significantly greater in the active group (adjusted mean weekly difference between groups = 5.71, 95% CI: 0.62‐10.81, p=0.028). The changes in all four TCQ domain scores also indicate significant differences between groups following the target quit date, which were durable for the expectancy, compulsivity and purposefulness domains, but not for the emotionality domain (see supplementary information).
At the first treatment visit, craving VAS scores following provocation increased in both groups (before the rTMS session), but the reduction in craving following rTMS (VAS3 minus VAS2) in the active group was significantly greater than in the sham group (F1,253=4.85, p=0.028) (see Figure 3). Of note, this acute reduction in craving (VAS3 minus VAS2 in the first treatment visit) significantly predicted eventual quitting in the active, but not the sham, group (odds ratio: active = 1.57, p=0.004; sham = 0.85, p=0.46). The effect of active rTMS on craving was also noted when comparing VAS1 scores on the second vs. the first day of treatment, or over all treatment visits (see Figure 4).
Figure 3.
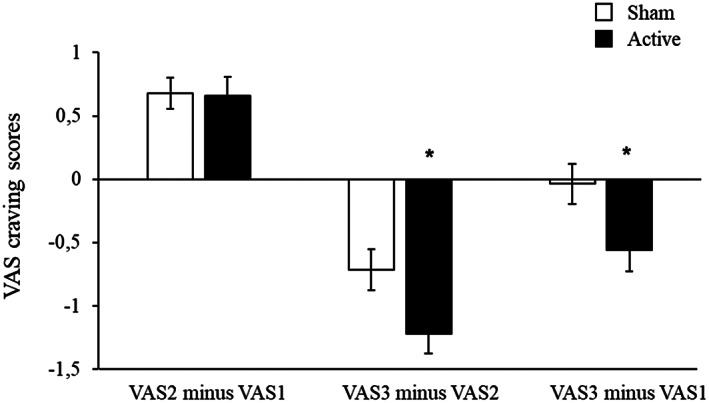
Acute changes in Visual Analogue Scale (VAS) craving scores following provocation (VAS2 minus VAS1) and following repetitive transcranial magnetic stimulation (VAS3 minus VAS2) in patients receiving active or sham treatment in the first session. Overall changes in craving during the first session (VAS3 minus VAS1) indicate that craving in the sham group returns to baseline, whereas it is reduced in the active group (F1,253=5.00, p=0.026). *p<0.05.
Figure 4.
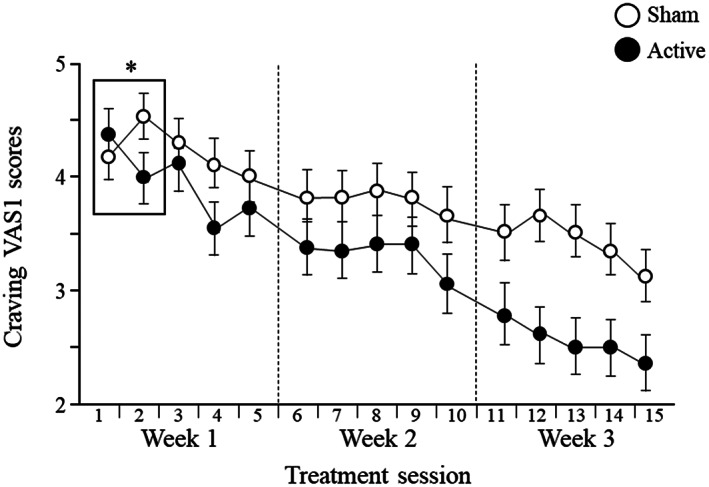
Daily changes in baseline craving (VAS1) scores during the first three weeks of treatment in patients receiving active or sham repetitive transcranial magnetic stimulation. ANOVA comparing VAS1 scores on the second vs. the first day of treatment (see box) revealed a significant interaction effect (F1,165=3.70, p=0.025). Repeated measure ANOVA during the treatment period revealed main effects for group (F1,159=4.50, p=0.035) and time (F14,2226=16.79, p<0.0001), as well as for group x time interaction (F14,2226=1.79, p=0.034). *p<0.05.
No statistically significant differences between the groups were detected for the change in FTND (dependence) or MNWS self‐report or observer‐report (withdrawal symptoms) scores (see supplementary information).
Safety analysis and blinding
No differences between groups were observed in vital signs, weight or cognition (measured by the MMSE and BSRT) at any time point (see supplementary information). The blinding assessment (in which subjects were asked to guess whether they received active or sham treatment) indicated that the majority of subjects in each group did not know which treatment they received, with no significant difference between the groups (p=0.65).
Adverse events were typical to those of similar rTMS systems and other TMS devices and were at least comparable to those of medications21, 36, 37, 38. The most frequent adverse event was headache (24.4% and 18.0% in the active and sham groups, respectively). Various forms of pain or discomfort (application site pain/discomfort, pain in jaw, facial pain, muscle pain/spasm/twitching, neck pain) were usually reported as either mild or moderate and resolved after treatment. In most of the participants the discomfort or pain disappeared once the participants became accustomed to the treatment.
Although a significant difference was found between the active and sham groups concerning the proportion of participants reporting any adverse event (53.7% vs. 36.0%, X2=8.274, p=0.004), there were no significant differences between the treatment groups for any specific adverse event, except for application site discomfort (see supplementary information).
One serious adverse event of tinnitus (which resolved) was reported as possibly related to treatment, and participation was terminated by the investigator. The drop‐out rate (at Week 6) was 39% for the active group and 32% for the sham group, without a significant difference between groups.
DISCUSSION
This study is the first large multicenter RCT to examine the safety and efficacy of brain stimulation in addiction medicine. We found that three weeks of daily rTMS targeting the lateral prefrontal cortex and insula during cue‐induced craving, followed by once weekly rTMS for three weeks, was a safe and effective intervention in chronic smokers with a DSM‐5 diagnosis of tobacco use disorder who had made at least one prior failed attempt to quit (with 68% having made at least three failed attempts). Active treatment more than doubled the quit rate and significantly reduced craving and cigarette consumption, relative to sham control.
Since there are no previous medical devices that aid smoking cessation, the safety and efficacy of this treatment can only be compared to those of FDA‐approved medications, including bupropion and varenicline38. Yet, there are several limitations to such comparison, as the sample sizes were larger and the follow‐up period longer in the pharmacological studies than in the current one. On the other hand, confirmatory testing in most those studies was done using exhaled breath testing for carbon monoxide levels rather than urine testing for cotinine levels, therefore confirming abstinence for a duration of hours instead of days.
In this study, the safety profile was not worse than smoking cessation medications and was similar to that observed in other multicenter rTMS trials, while efficacy was at least similar to medications in terms of relative improvement and effect sizes (active vs. sham). For example, in the bupropion studies, the quit rates of the treatment groups (300 mg/day) were 28% vs. 16% for placebo from Week 4 to 735, or 44% vs. 19% for placebo at Week 733. In another study32, bupropion, varenicline and placebo induced an abstinence rate from Week 9 to 12 of 29%, 44%, and 18%, respectively. As stated, those studies did not use urine testing for cotinine levels.
A recent large‐scale study which utilized urine cotinine levels as an objective measure for confirming abstinence (as in the present study, rather than just exhaled carbon monoxide measures), found that the most effective intervention – including both medications and monetary incentives – produced a 6‐month sustained abstinence rate of 12.7% among actively‐engaged and motivated participants, while the abstinence rate among those receiving smoking cessation medications without monetary incentives was only 2.9%9.
An important feature of our trial was the combination of the pre‐rTMS provocation and the post‐rTMS motivational talk (in both active and sham groups), although we did not test whether and to what degree these were necessary for the rTMS therapeutic effect. However, previous studies suggest that activation of the addiction circuitry by provocation makes it more amenable to modulation, where rTMS may open a “plasticity window” and behavioral intervention can be more effective39.
In our study, craving levels of both groups were equally affected by the provocation at the first visit, but active rTMS targeting the lateral prefrontal cortex and insula led to greater acute reduction of VAS craving scores, and the magnitude of this reduction predicted eventual quitting. A possible interpretation for this finding is that effective interference with an activated craving circuit may be an important element in the rTMS mechanism for addiction treatment, and that individual’s neural excitability in these regions following induction of craving may affect the clinical outcomes.
The suggested direct influence of rTMS on these brain areas is further highlighted by the attributed role of the lateral prefrontal cortex and insula in functions measured by the TCQ domains. Both areas are implicated in anticipation of rewarding outcomes (expectancy), intention to smoke (purposefulness), and control over use (compulsivity)40, 41, while the emotionality domain is more restricted to the insular cortex, which – due to its deeper location – may require higher rTMS dosage to implement long‐term modifications42. All these TCQ domains were significantly affected by active as compared to sham treatment in our trial.
In conclusion, this study extends the evidence supporting the use of rTMS for the treatment of substance use disorders by showing that it is a safe and effective treatment for tobacco use disorder. The trial represents the first large multicenter RCT of brain stimulation in addiction medicine and has led to the first clearance by the FDA for rTMS as an aid in smoking cessation.
This study suggests that rTMS directly affects neurocircuitry implicated in craving and might be effective in treating other addictions as well. The clinical benefits, including the fast onset and minor side effects, outweigh the minimal risks involved. The treatment may be particularly of help in patients with a DSM‐5 diagnosis of tobacco use disorder who have a long history of smoking and have made several failed attempts to quit using currently available options.
ACKNOWLEDGEMENTS
This trial was financially supported by an unrestricted grant from Brainsway Ltd. This company had no role in data analysis, that was conducted by an independent agency (A. Stein ‐ Regulatory Affairs Consulting and Biostats). A. Zangen and Y. Roth are inventors of deep TMS coils and have financial interest in Brainsway Ltd. A. Tendler is the chief medical officer of Brainsway Ltd. M.S. George is a member of the Brainsway Scientific Advisory Board (uncompensated) and his role as co‐principal investigator of the trial was uncompensated. A. Zangen and M.S. George are joint corresponding authors of this paper. The authors would like to thank K. Brady and K. Hartwell for help with initial design of the study and training of investigators in the symptom provocation and motivational talks. Supplementary information on the study is available at https://itonline.co.il/data/.
REFERENCES
- 1.Ziemann U.Thirty years of transcranial magnetic stimulation: where do we stand? Exp Brain Res 2017;235:973‐84. [DOI] [PubMed] [Google Scholar]
- 2.Roth Y, Zangen A, Hallett M. A coil design for transcranial magnetic stimulation of deep brain regions. J Clin Neurophysiol 2002;19:361‐70. [DOI] [PubMed] [Google Scholar]
- 3.Carmi L, Tendler A, Bystritsky A et al. Efficacy and safety of deep transcranial magnetic stimulation for obsessive‐compulsive disorder: a prospective multicenter randomized double‐blind placebo‐controlled trial. Am J Psychiatry 2019;176:931‐8. [DOI] [PubMed] [Google Scholar]
- 4.Levkovitz Y, Isserles M, Padberg F et al. Efficacy and safety of deep transcranial magnetic stimulation for major depression: a prospective multicenter randomized controlled trial. World Psychiatry 2015;14:64‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Reardon JP, Solvason HB, Janicak PG et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry 2007;62:1208‐16. [DOI] [PubMed] [Google Scholar]
- 6.Uhl GR, Koob GF, Cable J. The neurobiology of addiction. Ann NY Acad Sci 2019;1451:5‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gowing LR, Ali RL, Allsop S et al. Global statistics on addictive behaviours: 2014 status report. Addiction 2015;110:904‐19. [DOI] [PubMed] [Google Scholar]
- 8.American Psychiatric Association . Diagnostic and statistical manual of mental disorders, 5th ed. Arlington: American Psychiatric Association, 2013. [Google Scholar]
- 9.Halpern SD, Harhay MO, Saulsgiver K et al. A pragmatic trial of e‐cigarettes, incentives, and drugs for smoking cessation. N Engl J Med 2018;378:2302‐10. [DOI] [PubMed] [Google Scholar]
- 10.Benowitz NL. Nicotine addiction. N Engl J Med 2010;362:2295‐303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodchild M, Nargis N, d’Espaignet ET. Global economic cost of smoking‐attributable diseases. Tob Control 2018;27:58‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amiaz R, Levy D, Vainiger D et al. Repeated high‐frequency transcranial magnetic stimulation over the dorsolateral prefrontal cortex reduces cigarette craving and consumption. Addiction 2009;104:653‐60. [DOI] [PubMed] [Google Scholar]
- 13.Eichhammer P, Johann M, Kharraz A et al. High‐frequency repetitive transcranial magnetic stimulation decreases cigarette smoking. J Clin Psychiatry 2003;64:951‐3. [DOI] [PubMed] [Google Scholar]
- 14.Levy D, Shabat‐Simon M, Shalev U et al. Repeated electrical stimulation of reward‐related brain regions affects cocaine but not “natural” reinforcement. J. Neurosci 2007;27:14179‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li X, Hartwell KJ, Owens M et al. Repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex reduces nicotine cue craving. Biol Psychiatry 2013;73:714‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wing VC, Bacher I, Wu BS et al. High frequency repetitive transcranial magnetic stimulation reduces tobacco craving in schizophrenia. Schizophr Res 2012;1:264‐6. [DOI] [PubMed] [Google Scholar]
- 17.Strafella AP, Paus T, Barrett J et al. Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J Neurosci 2001;21:RC157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zangen A, Hyodo K. Transcranial magnetic stimulation induces increases in extracellular levels of dopamine and glutamate in the nucleus accumbens. Neuroreport 2002;13:2401‐5. [DOI] [PubMed] [Google Scholar]
- 19.Dinur‐Klein L, Dannon P, Hadar A et al. Smoking cessation induced by deep repetitive transcranial magnetic stimulation of the prefrontal and insular cortices: a prospective, randomized controlled trial. Biol Psychiatry 2014;76:742‐9. [DOI] [PubMed] [Google Scholar]
- 20.Hauer L, Scarano GI, Brigo F et al. Effects of repetitive transcranial magnetic stimulation on nicotine consumption and craving: a systematic review. Psychiatry Res 2019;281:112562. [DOI] [PubMed] [Google Scholar]
- 21.Ekhtiari H, Tavakoli H, Addolorato G et al. Transcranial electrical and magnetic stimulation (tES and TMS) for addiction medicine: a consensus paper on the present state of the science and the road ahead. Neurosci Biobehav Rev 2019;104:118‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keel JC, Smith MJ, Wassermann EM. A safety screening questionnaire for transcranial magnetic stimulation. Clin Neurophysiol 2001;112:720. [DOI] [PubMed] [Google Scholar]
- 23.Heishman SJ, Singleton EG, Moolchan ET. Tobacco Craving Questionnaire: reliability and validity of a new multifactorial instrument. Nicotine Tob Res 2003;5:645‐54. [DOI] [PubMed] [Google Scholar]
- 24.Heatherton TF, Kozlowski LT, Frecker RC et al. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict 1991;86:1119‐27. [DOI] [PubMed] [Google Scholar]
- 25.Bramer SL, Kallungal BA. Clinical considerations in study designs that use cotinine as a biomarker. Biomarkers 2003;8:187‐203. [DOI] [PubMed] [Google Scholar]
- 26.Schick SF, Blount BC, Jacob P 3rd et al. Biomarkers of exposure to new and emerging tobacco delivery products. Am J Physiol Lung Cell Mol Physiol 2017;313:L425‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hughes JR. Tobacco withdrawal in self‐quitters. J Consult Clin Psychol 1992;60:689‐97 [DOI] [PubMed] [Google Scholar]
- 28.Folstein MF, Folstein SE, McHugh PR. “Mini‐Mental State”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189‐98. [DOI] [PubMed] [Google Scholar]
- 29.Buschke H.Selective reminding for analysis of memory and learning. J Verb Learning Verb Behav 1973;12:543‐50. [Google Scholar]
- 30.Fiocchi S, Chiaramello E, Luzi L et al. Deep transcranial magnetic stimulation for the addiction treatment: electric field distribution modeling. IEEE J Electromagn RF Microw Med Biol 2018;2:242‐8. [Google Scholar]
- 31.National Cancer Institute . Clearing the air. https://www.cancer.gov.
- 32.Gonzales D, Rennard S, Nides M et al. Varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, vs sustained‐release bupropion and placebo for smoking cessation. A randomized controlled trial. JAMA 2006;296:47‐55. [DOI] [PubMed] [Google Scholar]
- 33.Hurt RD, Sachs DP, Glover ED et al. A comparison of sustained‐release bupropion and placebo for smoking cessation. N Engl J Med 1997;337:1195‐202. [DOI] [PubMed] [Google Scholar]
- 34.Jorenby DE, Leischow SJ, Nides MA et al. A controlled trial of sustained‐release bupropion, a nicotine patch, or both for smoking cessation. N Engl J Med 1999;340:685‐91. [DOI] [PubMed] [Google Scholar]
- 35.Tashkin D, Kanner R, Bailey W et al. Smoking cessation in patients with chronic obstructive pulmonary disease: a double‐blind, placebo‐controlled, randomised trial. Lancet 2001;357:1571‐5. [DOI] [PubMed] [Google Scholar]
- 36.Lefaucheur J‐P, André‐Obadia N, Antal A et al. Evidence‐based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin Neurophysiol 2014;125:2150‐206. [DOI] [PubMed] [Google Scholar]
- 37.Zibman S, Pell GS, Barnea‐Ygael N et al. Application of transcranial magnetic stimulation for major depression: coil design and neuroanatomical variability considerations. Eur Neuropsychopharmacol 2021;45:73‐88. [DOI] [PubMed] [Google Scholar]
- 38.Food and Drug Administration . FDA revises description of mental health side effects of the stop‐smoking medicines Chantix (varenicline) and Zyban (bupropion) to reflect clinical trial findings. https://www.fda.gov.
- 39.Tendler A, Sisko E, Barnea‐Ygael N et al. A method to provoke obsessive compulsive symptoms for basic research and clinical interventions. Front Psychiatry 2019;10:814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moran LV, Stoeckel LE, Wang K et al. Nicotine increases activation to anticipatory valence cues in anterior insula and striatum. Nicotine Tob Res 2018;20:851‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Janes AC, Nickerson LD, Frederick BD et al. Prefrontal and limbic resting state brain network functional connectivity differs between nicotine‐dependent smokers and non‐smoking controls. Drug Alcohol Depend 2012;125:252‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Versace F, Engelmann JM, Jackson EF et al. Do brain responses to emotional images and cigarette cues differ? An fMRI study in smokers. Eur J Neurosci 2011;34:2054‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]


