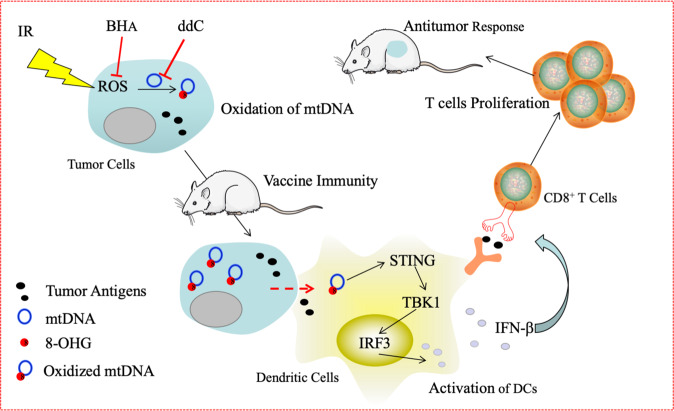
Fig. 8.
Oxidized mitochondrial DNA from irradiated tumor cells gains access to the cytoplasm of dendritic cells, subsequently activating the STING-TBK1-IRF3-type I interferon pathway and eliciting antitumor immunity. Irradiation induces tumor cell apoptosis, ROS release, and upregulation of the 8-OHG content, which results in oxidative mtDNA damage in tumor cells. After immunization with an irradiated tumor cell vaccine, oxidized mtDNA derived from tumor cells is transferred to the cytoplasm of DCs, which in turn activates STING-TBK1-IRF3 signaling in the DC cytoplasm and induces type I interferon production. Type I interferons enhance the cross-presentation of apoptotic tumor cell-derived antigens after irradiation and potentiate the proliferation of CD8+ T cells, leading to protective antitumor immunity. Moreover, a ROS scavenger (BHA) and an inhibitor of mtDNA polymerase γ (ddC) have the ability to impair CD8+ T cell proliferation by inhibiting oxidative mtDNA damage