Abstract
Purpose:
To determine the incidence of and predictive factors for cataract in intermediate uveitis.
Design:
Retrospective cohort study
Methods:
Patients were identified from the Systemic Immunosuppressive Therapy for Eye Diseases (SITE) Cohort Study, in which medical records were reviewed to determine demographic and clinical data of every eye/patient at every visit at five participating United States tertiary care uveitis centers. The primary outcome was development of vision-compromising cataract as defined by a decrease in visual acuity to 20/40 or less, or requiring cataract surgery. Survival analysis assessed visually defined cataract to avoid bias due to timing of surgery vis-à-vis inflammatory status.
Results
Among 2,190 eyes of 1,302 patients with intermediate uveitis the cumulative incidence of cataract formation was 7.6% by one year (95% CI=6.2–9.1%), increasing to 36.6% by ten years (95% CI=31.2–41.6%). Increased cataract risk was observed in eyes with concurrent anterior uveitis causing posterior synechiae (HR=2.68, 95% CI=2.00–3.59, p<0.001), and in eyes with epiretinal membrane formation (HR=1.54, 95% CI=1.15–2.07, p=0.004). Higher dose corticosteroid therapy was associated with significantly higher incidence of cataract, especially time-updated use of topical corticosteroids ≥2 times/day or ≥4 periocular corticosteroid injections. Low dose corticosteroid medications (oral prednisone 7.5mg daily or less, or topical corticosteroid drops <2 times/day) were not associated with increased cataract risk.
Conclusions:
Our study found that the incidence of clinically important cataract in intermediate uveitis is moderate. The risk is higher with markers of severity, and with higher doses of corticosteroid medications, the latter being potentially modifiable.
Introduction
Uveitis represents a heterogeneous group of inflammatory conditions affecting the eye, and is estimated to be responsible for as much as 10–15% of visual loss in the adult population worldwide.1 Cataracts are among the complications of uveitis which can impair vision.1 Uveitic cataracts may require surgery to improve visual function, prevent further inflammation, or allow improved clinical examination for treatment and monitoring.1,2,3 Studies have shown that controlling intraocular inflammation reduces the incidence of cataracts.4 The primary treatments used in managing uveitis also are potentially cataractogenic.4,5 Therefore, prompt treatment of intraocular inflammation while minimizing corticosteroid exposure is of the utmost importance for preventing complications of uveitis and vision loss.
Within the broader category of uveitis, patients can be categorized according to the primary site of inflammation within the eye; intermediate uveitis refers to inflammation that primarily affects the vitreous.6 Intermediate uveitis constitutes 2–31% of all uveitis and cataract has been reported to be one of the most common complications in this disease.5 Patients with intermediate uveitis often require systemic immunomodulatory therapy and close monitoring given the potential for exacerbations, but with proper and timely treatment good visual acuity can be maintained.5 Indeed, after seven years of follow up in the Multicenter Uveitis Steroid Treatment (MUST) Trial, patients treated with systemic anti-inflammatory therapy, including those with intermediate uveitis, maintained their baseline visual acuity on average.7
Uveitic cataracts pose a particular challenge for the surgeon as eyes with prior inflammation are prone to intraocular scarring, poor pupillary dilation, abnormally fragile iris vasculature, and repeat inflammation in the perioperative period.4,8 These concerns apply in the subset of patients with intermediate uveitis as well. However, the situation regarding cataract incidence and its treatment may differ from anterior uveitis because the primary site of inflammation is behind rather than around the lens. Understanding the risks of cataract formation and need for cataract surgery in intermediate uveitis can help guide treatment and inform patient counseling. In this study, we analyze the incidence of clinically significant cataract in a large cohort of eyes of patients with intermediate uveitis.
Methods
Study Population.
Cases of intermediate uveitis were identified from the Systemic Immunosuppressive Therapy for Eye Disease (SITE) Cohort Study, a large retrospective study of patients with noninfectious inflammatory eye disease examined at US tertiary subspecialty centers, which has been previously described.9,10 The study subsequently has been extended to include all eligible patients at the five centers from inception of the subspecialty uveitis practice at each through December 31, 2010. Those diagnosed with intermediate uveitis at the participating five core centers were included in this study. Intermediate uveitis had been defined as inflammation primarily localized to the vitreous, following the definition eventually agreed upon by the Standardization of Uveitis Nomenclature (SUN) Working Group.6 Patients with combined anterior and intermediate uveitis were included; special note was made of eyes with anterior uveitis or signs thereof, and analyses were adjusted for this factor. The Institutional Review Boards of the University of Pennsylvania, Oregon Health & Sciences University, Johns Hopkins School of Medicine, Massachusetts Eye and Ear, and the National Eye Institute approved the study, including waiver of informed consent for this retrospective cohort study, which involved no contact with human subjects. The study was conducted adhering to the tenets of the Declaration of Helsinki, and was compliant with all relevant laws.
Data Collection.
All data collected had been entered into a computer-based standardized data form developed specifically for the SITE study.9 This system included built-in quality control measures, and allowed for correction of data in real time. Data evaluated for this paper (see Table 1 and Table 2) included patients’ demographic characteristics, objective inflammatory findings, duration of uveitis, and previous treatments pursued, including medication use and prior surgeries. Whether or not patients were taking several different common systemic medications, including aspirin, statins, angiotensin converting enzyme (ACE) inhibitors, and nonsteroidal anti-inflammatory drugs (NSAIDs), also was noted in order to assess the hypotheses that each of these might modify the risk of cataract. Presence or absence of cataract in the fellow eye also was noted.
Table 1.
Demographic Characteristics, eyes of patients with intermediate uveitis, Systemic Immunosuppressive Therapy for Eye Diseases (SITE) Cohort Study.
| Cox model of Cataract by Visual Acuity | ||||||||
|---|---|---|---|---|---|---|---|---|
| Cataract | Crude | Adjusted* | ||||||
| Total | No | Yes | Hazard Ratio (95% CI) | p | Hazard Ratio (95% CI) | p | ||
| Age at presentation | <40 | 1344 (61%) | 1190 (89%) | 154 (11%) | Ref | 0.30 | Ref | 0.19 |
| 40–60 | 657 (30%) | 572 (87%) | 85 (13%) | 1.14 (0.84, 1.55) | 1.04 (0.76, 1.42) | |||
| >60 | 189 (9%) | 170 (90%) | 19 (10%) | 1.52 (0.86, 2.70) | 1.73 (0.96, 3.12) | |||
| Sex | Male | 785 (36%) | 696 (89%) | 89 (11%) | Ref | 0.90 | Ref | 0.89 |
| Female | 1405 (64%) | 1236 (88%) | 169 (12%) | 1.02 (0.76, 1.37) | 0.98 (0.73, 1.31) | |||
| Race category | White | 1569 (72%) | 1388 (88%) | 181 (12%) | Ref | 0.51 | Ref | 0.45 |
| Black | 219 (10%) | 186 (85%) | 33 (15%) | 1.26 (0.85, 1.86) | 1.30 (0.84, 1.99) | |||
| Hispanic | 83 (4%) | 75 (90%) | 8 (10%) | 0.73 (0.35, 1.53) | 0.81 (0.38, 1.75) | |||
| Other | 319 (15%) | 283 (89%) | 36 (11%) | 1.04 (0.70, 1.55) | 1.22 (0.83, 1.81) | |||
| Smoking | Never | 1311 (60%) | 1168 (89%) | 143 (11%) | Ref | 0.24 | Ref | 0.53 |
| Past | 203 (9%) | 175 (86%) | 28 (14%) | 1.23 (0.75, 2.00) | 1.06 (0.64, 1.75) | |||
| Current | 484 (22%) | 417 (86%) | 67 (14%) | 1.43 (1.01, 2.02) | 1.27 (0.90, 1.80) | |||
| Unknown | 192 (9%) | 172 (90%) | 20 (10%) | 1.11 (0.65, 1.87) | 0.91 (0.48, 1.72) |
Adjusted for PPV, posterior synechia, epiretinal membrane, any IMTs, cumulative periocular corticosteroid injections, topical corticosteroids, and oral corticosteroids.
CI = confidence interval; Ref = reference value
Table 2.
Selected clinical examination findings, eyes of patients with intermediate uveitis, Systemic Immunosuppressive Therapy for Eye Diseases (SITE) Cohort Study
| Cox model of Cataract by Visual Acuity | ||||||||
|---|---|---|---|---|---|---|---|---|
| Cataract | Crude | Adjusted* | ||||||
| Total | No | Yes | Hazard Ratio (95% CI) | p | Hazard Ratio (95% CI) | p | ||
| Anterior/Intermediate Uveitis at baseline | No | 1564 (72%) | 1411 (90%) | 153 (10%) | Ref | <0.001 | Ref | 0.75 |
| Yes | 622 (28%) | 517 (83%) | 105 (17%) | 1.76 (1.32, 2.34) | 1.05 (0.76, 1.46) | |||
| Bilateral Uveitis | No | 227 (10%) | 197 (87%) | 30 (13%) | Ref | 0.15 | Ref | 0.10 |
| Yes | 1963 (90%) | 1735 (88%) | 228 (12%) | 0.75 (0.50, 1.11) | 0.70 (0.46, 1.07) | |||
| Duration of uveitis prior to presentation | <6 Months | 816 (37%) | 747 (92%) | 69 (8%) | Ref | 0.06 | Ref | 0.19 |
| 6 Months to <2 Years | 507 (23%) | 443 (87%) | 64 (13%) | 1.35 (0.91, 2.01) | 1.32 (0.89, 1.95) | |||
| 2+ Years | 865 (40%) | 741 (86%) | 124 (14%) | 1.53 (1.08, 2.17) | 1.43 (0.97, 2.11) | |||
| Cataract in other eye** | No | 2008 (92%) | 1761 (88%) | 247 (12%) | Ref | 0.59 | Ref | 0.48 |
| Yes | 182 (8%) | 171 (94%) | 11 (6%) | 1.11 (0.76, 1.62) | 0.86 (0.56, 1.31) | |||
| Inflammatory Activity** | Inactive | 392 (18%) | 356 (91%) | 36 (9%) | Ref | 0.16 | Ref | 0.12 |
| Slightly active | 238 (11%) | 221 (93%) | 17 (7%) | 1.41 (0.97, 2.06) | 1.24 (0.84, 1.83) | |||
| Active | 1554 (71%) | 1349 (87%) | 205 (13%) | 1.00 (0.72, 1.38) | 0.82 (0.59, 1.14) | |||
| Epiretinal Membrane** | No | 2003 (92%) | 1775 (89%) | 228 (11%) | Ref | <0.001 | Ref | 0.004 |
| Yes | 180 (8%) | 151 (84%) | 29 (16%) | 1.91 (1.45, 2.51) | 1.54 (1.15, 2.07) | |||
| Posterior Synechia** | No | 1925 (88%) | 1725 (90%) | 200 (10%) | Ref | <0.001 | Ref | <0.001 |
| Yes | 258 (12%) | 200 (78%) | 58 (22%) | 3.01 (2.27, 3.99) | 2.68 (2.00, 3.59) | |||
| Pars Plana Vitrectomy (not for Retinal Detachment) | No | 2128 (97%) | 1880 (88%) | 248 (12%) | Ref | <0.001 | Ref | 0.004 |
| Yes | 62 (3%) | 52 (84%) | 10 (16%) | 2.26 (1.46, 3.51) | 1.92 (1.24, 2.98) |
Adjusted for PPV, posterior synechia, epiretinal membrane, any IMTs, cumulative periocular corticosteroid injections, topical corticosteroids, and oral corticosteroids
Time-updated
CI = confidence interval; IMT = immunomodulatory therapy; PPV = pars plana vitrectomy; Ref = reference value
Study Outcomes.
The primary outcome in this study was the incidence of cataract. Incidence of cataract was determined by eye (as opposed to by patient). Cataract was defined by either decreased visual acuity to worse than 20/40 that was attributed to the presence of cataract, or cataract requiring surgical removal (occurrence of cataract surgery).
Statistical Analyses.
The frequencies of patient demographic characteristics and ocular inflammatory findings were calculated. The incidence rate (overall risk) for vision-compromising cataract was determined by number of incident cataracts divided by the eye-time at risk for development of cataract, along with a 95% confidence interval (CI) based on the assumption of a Poisson distribution. Potentially predictive factors were assessed using Cox regression models to calculate crude and adjusted hazard ratios (HR and aHR) for each potentially associated factor, based only on the cataracts defined using the visual acuity definition. Occurrence of cataract surgery was not used as an outcome for predictive factor assessment in order to avoid biases regarding inflammatory status covariates related to clinicians’ typical practice of insisting that inflammation be quiet for three months before cataract surgery in these cases. Inflammatory exam findings and treatment regimens were time-updated for each visit based on the status at the visit before. Final regression models and adjusted hazard ratios were adjusted for history of pars plana vitrectomy, presence of posterior synechiae, presence of epiretinal membrane, and use of treatment with oral, topical, or periocular corticosteroids, as well as any other immunomodulatory therapy. To illustrate key findings, Kaplan-Meier curves were drawn to visually display crude incidence of cataract formation over time for a given risk factor. All statistical analyses were performed using SAS 9.4 (Cary, NC, USA).
Results
Two thousand one hundred ninety eyes (of 1,302 patients) met criteria for inclusion, and were followed over 6,317 eye-years (3,886 person-years) at risk of cataract according to the study definitions. The majority of the eyes with intermediate uveitis belonged to female patients (64%), white patients (72%), non-smokers (60%), and patients under the age of 40 (61%), including 463 (21%) pediatric patients younger than age 18. An additional 30% were between ages 40 and 60, and only 9% of patients were over the age of 60 at the beginning of follow-up. Twenty-eight percent of the patients included had findings of both anterior and intermediate uveitis at baseline. Almost all of the cohort (90%) had bilateral intermediate uveitis. Patient demographics are further detailed in Table 1.
Over the course of the study, a total of 258 eyes developed cataract that was blamed for reducing visual acuity to a level worse than 20/40 and/or required cataract surgery. The cumulative incidence rate was 7.6% (95% CI=6.2–9.1%) by one year. The proportion developing cataract increased with longer follow up time to 12.8% (95% CI=10.8–14.7%) by two years, 24.1% (95% CI=20.9–27.2%) by five years, and 36.6% (95% CI=31.2–41.6%) by ten years (Figure 1).
Figure 1.
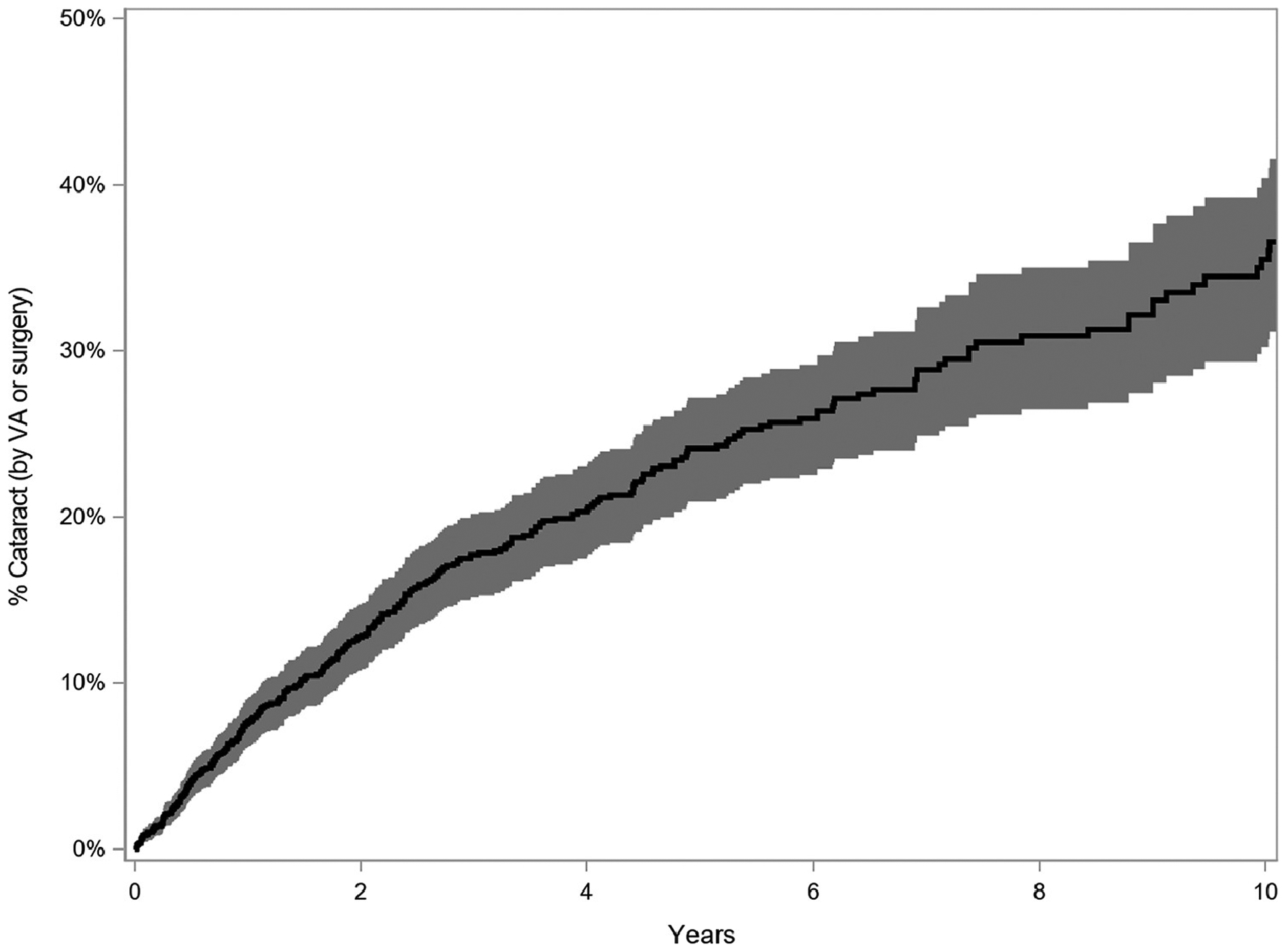
Kaplan Meier curve depicting the cumulative incidence of cataract formation (reduced visual acuity to worse than 20/40 attributed to cataract or occurrence of cataract surgery), with 95% confidence interval, among eyes of patients with intermediate uveitis, Systemic Immunosuppressive Therapy for Eye Diseases (SITE) Cohort Study
The observed incidence of cataract formation was not significantly associated with race, sex, bilaterality of intermediate uveitis, or smoking status. Neither were the systemic medications hypothesized to be associated with cataract risk (NSAIDs, ACE inhibitors, statins, and aspirin) associated with incident cataract formation (see Supplemental Table 1, available online, for further details). While not statistically significant, increasing patient age at presentation (aHR for patients over 60=1.73, 95% CI=0.96–3.12, p=0.19), and longer duration of uveitis (aHR for over 2 years=1.43, 95% CI=0.97–2.11, p=0.19) tended toward an increased risk of cataract formation. Incidence tended to increase with age >60 years at the beginning of follow-up, but only 9% were in this age range at cohort entry.
The study examined several time-updated measures of current activity of inflammation including anterior chamber cell, vitreous cell, vitreous haze, keratic precipitates, snowballs, snowbanking, and an overall grading of inflammatory activity as active, slightly active, or inactive. These measures were time-updated over the course of observation. None of these were associated with incident cataract in this intermediate uveitis population (as further detailed in Supplemental Table 2, available online).
When posterior synechiae, a marker of severity of prior or current anterior inflammation, were present in our cases of intermediate uveitis, the risk of cataract incidence was increased (see Table 2). Two hundred fifty-eight patients included in the study (12% of the study sample) had posterior synechiae on examination, and of these, 58 (22%) developed vision-compromising cataracts (aHR=2.68, 95% CI=2.00–3.59, p<0.001, Figure 2).
Figure 2.
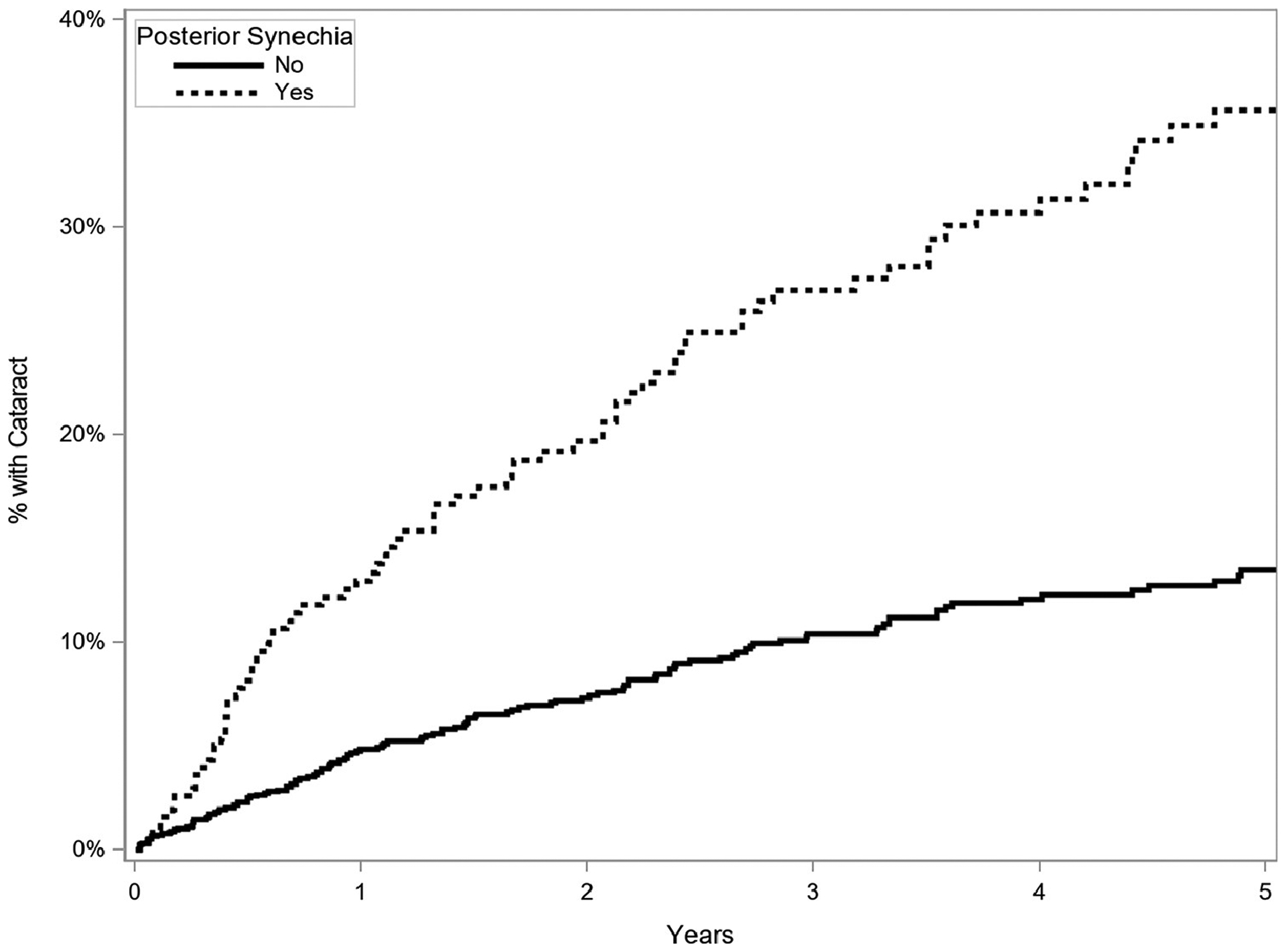
Kaplan Meier curve of incidence of cataract (reduced visual acuity to worse than 20/40 attributed to cataract or occurrence of cataract surgery) in eyes with and without presence of posterior synechiae (time-updated, once present always present), eyes of patients with intermediate uveitis, Systemic Immunosuppressive Therapy for Eye Diseases (SITE) Cohort Study
The presence of an epiretinal membrane (ERM), present in 180 eyes (8%), also was a statistically significant risk factor predicting incidence of cataract (aHR = 1.54, 95% CI=1.15–2.07, p=0.004, Table 2). Sixty-two eyes had a history of pars plana vitrectomy (PPV), which was also a statistically significant factor conferring increased risk of incident cataract (aHR=1.92, 95% CI=1.24–2.98, p=0.004, Table 2).
Anterior uveitis co-existent with intermediate uveitis was associated with increased crude incidence of cataract (HR=1.76, 95% CI=1.32–2.34, p<0.001), but association was abrogated by adjustment for other variables, including the presence of posterior synechiae (aHR=1.05, 95% CI=0.76–1.46, p=0.75) which typically is an indicator of past or present anterior inflammation (see Table 2).
Regarding anti-inflammatory treatments, time-updated use of topical, periocular, and oral corticosteroid was studied (Table 3). For topical corticosteroids (Figure 3), relative to no topical corticosteroid treatment, there was no increased incidence of cataract formation at a dose of 1 drop daily (aHR=0.79, 95% CI=0.34–1.82, p=0.01), but there was a statistically significantly increased risk with 2 or more drops per day (adjusted HR=1.55, 95% CI=1.13–2.12, p=0.01). For oral corticosteroids, there was a borderline decreased risk for prednisone (or equipotent dose of alternative corticosteroids) up to 7.5mg daily (adjusted HR=0.49, 95% CI=0.24–0.99), but no significant change in risk with doses over 7.5mg a day (aHR=1.32, 95% CI=0.92–1.90). For eyes treated with periocular injections of corticosteroids (Figure 4), those eyes which had undergone four or more cumulative injections had an increased risk of cataract (aHR=2.01, 95% CI=1.05–3.85, p=0.049); those eyes which had one (aHR=1.47, 95% CI=0.98–2.20) or two to three cumulative injections (aHR=1.42, 95% CI=0.86–2.33) tended to have higher risk of cataract than those not treated with periocular corticosteroid injections, but not to a statistically significant degree. Intraocular corticosteroid treatments were not often used during the period of observation and therefore were not assessed. Immunomodulatory therapy (IMT) use was associated with an increased risk of cataract (aHR=1.67, 95% CI=1.24–2.25, p<0.001).
Table 3.
Anti-inflammatory treatments, eyes of patients with intermediate uveitis, Systemic Immunosuppressive Therapy for Eye Diseases (SITE) Cohort Study
| Cox model of Cataract by Visual Acuity | ||||||||
|---|---|---|---|---|---|---|---|---|
| Cataract | Crude | Adjusted* | ||||||
| Total | No | Yes | Hazard Ratio (95% Cl) | p | Hazard Ratio (95% Cl) | p | ||
| Cumulative Periocular Corticosteroid Injections** | Never | 2062 (94%) | 1828 (89%) | 234 (11%) | Ref | 0.003 | Ref | 0.049 |
| 1 injection | 128 (6%) | 104 (81%) | 24 (19%) | 1.50 (1.04, 2.19) | 1.47 (0.98, 2.20) | |||
| 2–3 injections*** | 1.43 (0.88, 2.32) | 1.42 (0.86, 2.33) | ||||||
| ≥4 injections*** | 2.61 (1.45, 4.70) | 2.01 (1.05, 3.85) | ||||||
| Topical Corticosteroids** | None | 1327 (61%) | 1181 (89%) | 146 (11%) | Ref | <0.001 | Ref | 0.01 |
| 1 drop | 52 (2%) | 45 (87%) | 7 (13%) | 1.10 (0.45, 2.68) | 0.79 (0.34, 1.82) | |||
| ≥2 drops | 811 (37%) | 706 (87%) | 105 (13%) | 2.07 (1.54, 2.78) | 1.55 (1.13, 2.12) | |||
| Oral Corticosteroids** | None | 1834 (84%) | 1633 (89%) | 201 (11%) | Ref | 0.003 | Ref | 0.02 |
| >0 – ≤7.5 mg/day | 45 (2%) | 43 (96%) | 2 (4%) | 0.65 (0.30, 1.40) | 0.49 (0.24, 0.99) | |||
| >7.5 mg/day | 311 (14%) | 256 (82%) | 55 (18%) | 1.72 (1.22, 2.45) | 1.32 (0.92, 1.90) | |||
| Any IMT** | No | 1975 (90%) | 1746 (88%) | 229 (12%) | Ref | <0.001 | Ref | <0.001 |
| Yes | 215 (10%) | 186 (87%) | 29 (13%) | 1.90 (1.42, 2.55) | 1.67 (1.24, 2.25) |
Adjusted for PPV, posterior synechia, epiretinal membrane, any IMTs, cumulative periocular steroid injections, topical steroids, and oral steroids
Time-updated
No eyes met criteria for inclusion in these categories at time of enrollment, but were included as appropriate with time-updated data
CI = confidence interval; IMT = immunomodulatory therapy; PPV = pars plana vitrectomy; Ref = reference value
Figure 3.
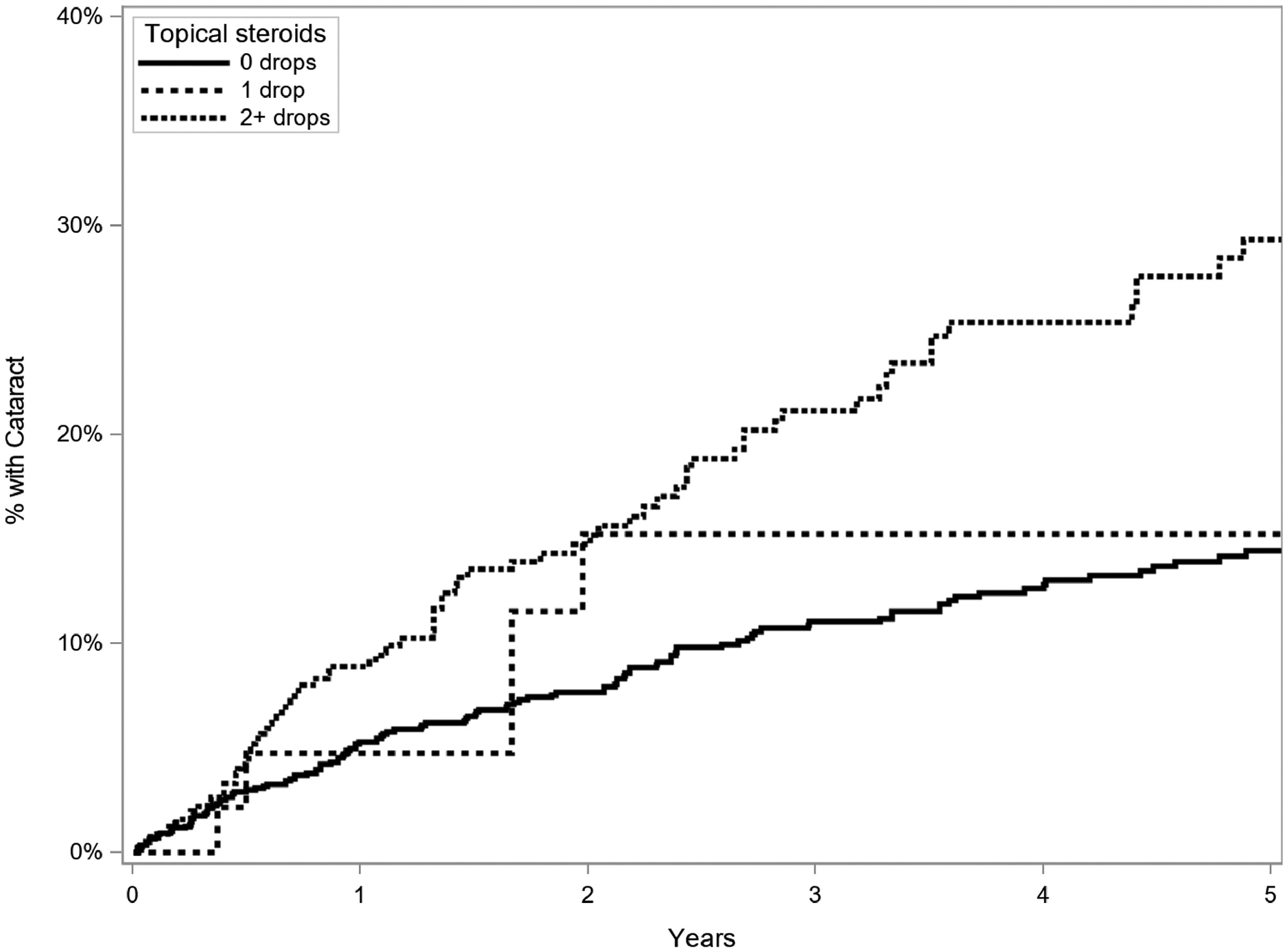
Kaplan Meier curve of incidence of cataract (reduced visual acuity to worse than 20/40 attributed to cataract or occurrence of cataract surgery) in eyes treated and not treated with topical corticosteroids (time-updated), eyes of patients with intermediate uveitis, Systemic Immunosuppressive Therapy for Eye Diseases (SITE) Cohort Study
Figure 4.
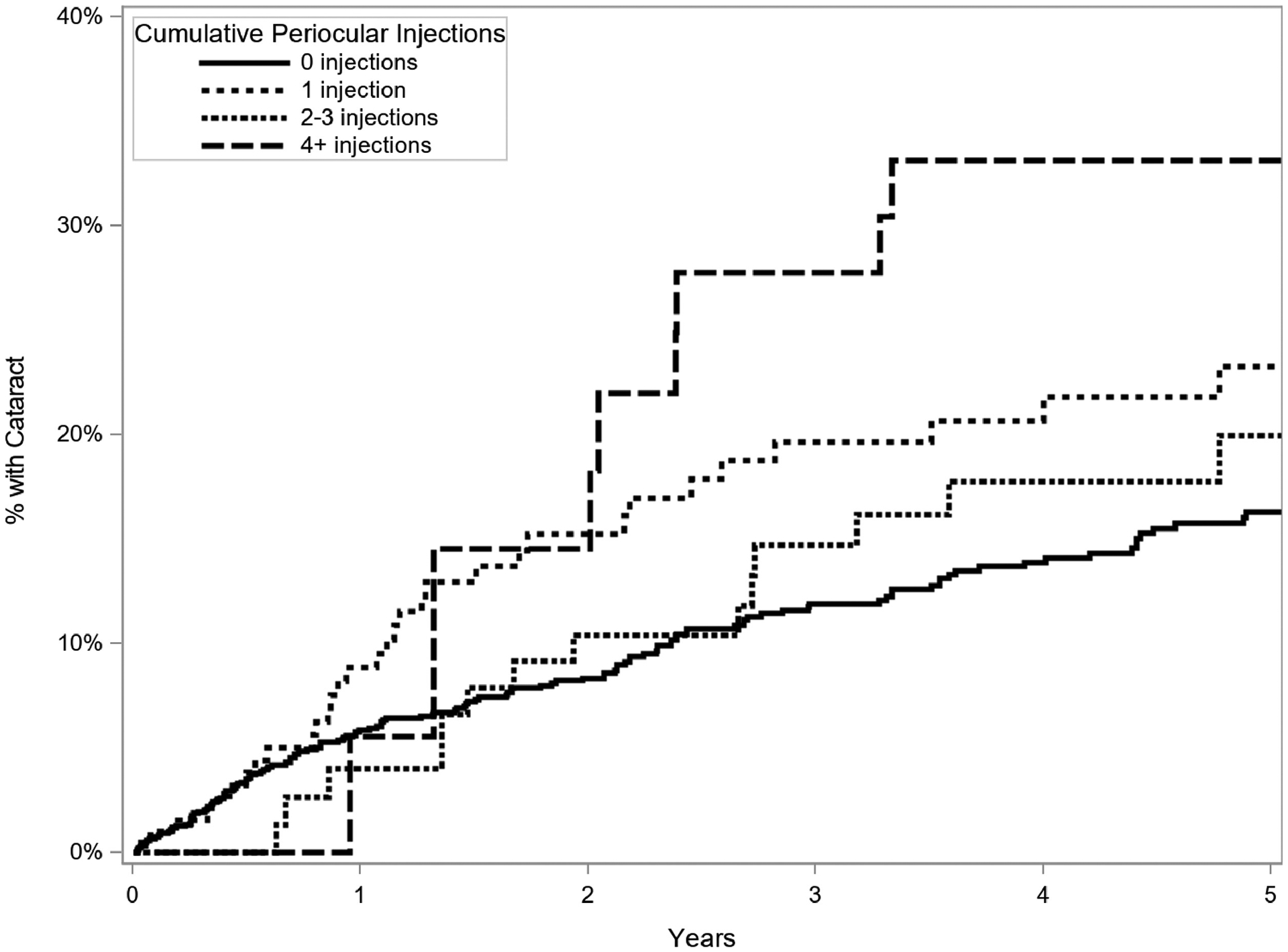
Kaplan Meier curve of incidence of cataract formation in eyes treated and not treated with periocular corticosteroid injections (time-updated cumulative number of injections), eyes of patients with intermediate uveitis, Systemic Immunosuppressive Therapy for Eye Diseases (SITE) Cohort Study
Discussion
In this large retrospective cohort of patients with intermediate uveitis there was a moderate absolute risk of cataract formation despite the younger age of most of the patients. Previous studies investigating cataracts and intermediate uveitis have either analyzed incident cataract formation in uveitis in general with intermediate uveitis only as a subgroup or have observed the prevalence (not incidence) of cataract in eyes with intermediate uveitis. These observational studies have found cataract to be a common complication of intermediate uveitis, affecting approximately one-quarter to one-third of intermediate uveitis patients generally over study periods of about eight to ten years, with good overall visual prognosis with surgery.3,5,11 The moderate risk of cataract formation observed in this study is consistent with these prior findings, but characterizes the incidence (and not just the prevalence) of cataract formation in patients with the intermediate uveitis type of uveitis specifically.
The overall demographic makeup of this study population is consistent with prior studies of the intermediate uveitis population.5,11,12 None of the patient-level characteristics analyzed (age, sex, race, and smoking status) were statistically significantly associated with an increased or decreased risk of cataract formation. Increasing patient age did tend towards a higher rate of cataract formation, but may have been a less important factor since few of our patients were in the age range where cataract commonly occurs.13
Time-updated degree of inflammatory activity was assessed, as described above. None of these time-updated markers of current inflammation were statistically significantly associated with a difference in incidence of cataract. This pattern of findings lies in contrast to prior studies of broader or different uveitis populations, which have demonstrated a higher risk of uveitis complications, including cataract, with increased inflammatory activity,3,14,15 but is in keeping with other studies that demonstrate a better visual prognosis with fewer complications in patients with intermediate uveitis as compared to those with more posterior uveitis or panuveitis.12 Increased duration of uveitis prior to the beginning of observation tended to be associated with higher risk, but also not to a statistically significant degree, in contrast to previous studies of broader or different uveitis populations.3,14 Given that the site of inflammation in intermediate uveitis is predominantly in the vitreous (except when there is co-existing anterior uveitis), the adverse effects of inflammation itself on the lens may be less than with anterior uveitis where the inflammation is centered in the adjacent ciliary body and/or iris. The lack of association between intermediate uveitis inflammatory activity and cataract formation as compared to the association found between active inflammation and cataract in other uveitis populations is surprising and warrants further investigation. Association between modest intermediate uveitis inflammatory activity and cataract would call into question the practice of simply observing low grade intermediate uveitis activity; our study did not detect such an association in intermediate uveitis cases unless anterior uveitis also was present.
Cases of combined anterior and intermediate uveitis included in this analysis had a higher risk of cataract which appeared to be mediated through related factors such as presence of posterior synechiae, which was strongly associated with cataract in this analysis, as we also observed posterior synechiae as a risk factor in an anterior uveitis population (not shown). Posterior synechiae tended to be a stronger predictor than anterior synechiae, consistent with other prior studies.15,16,17 The limited associations with current inflammation we observed may reflect that direct effects of inflammation may occur primarily in the intermediate uveitis group with associated anterior uveitis, and that this effect is best captured by the presence of posterior synechiae (see below) among intermediate uveitis cases (see below). We hypothesize that mechanical effects on the lens might contribute to increased cataract risk over and above the extent to which posterior synechiae represent a more severe inflammatory experience.
The presence of epiretinal membrane also was significantly associated with increased incidence of cataract formation in this cohort, which also may be a marker of cumulative disease severity,11,15,16,17,18,19 which might have more efficiently captured the cumulative impact of inflammation than current (time-updated) inflammatory status in this analysis. While ascertainment of ERM and its relative contribution to visual decline compared to cataract in the same eye is inherently limited in a chart review study, the SITE database included attribution of the primary cause of each patient’s visual impairment. When cataract was indicated, this was counted for our study. In a recent paper by Pistilli et al,20 patients in this cohort who underwent cataract surgery had, on average, a three line improvement in visual acuity, confirming clinician accuracy in their evaluations.
Similarly, use of IMT was a risk factor associated with cataract incidence in this cohort. Since treatment for intermediate uveitis is not always necessary in cases of mild inflammation limited to the vitreous,11,21 and IMT is generally started only in patients with more severe inflammation that is refractory to first-line corticosteroids,22 treatment with IMT may not have been an independent risk factor for cataract formation in this analysis. Rather, it may have served as a surrogate marker of disease severity, inflammation extending beyond the vitreous, and/or intermediate uveitis in association with systemic disease.
As expected, patients in this study with a history of pars plana vitrectomy (PPV) had a higher incidence of cataract formation. Vitrectomy is well known to cause or accelerate the rate of cataract formation.23 Vitrectomy is being considered as a potential remittive treatment for intermediate uveitis to prevent inflammation.24 If PPV is pursued to treat intermediate uveitis, the effects of vitrectomy on cataract formation should be considered in evaluating the potential benefit of inflammation remission.
Also in keeping with prior reports,3,7,25,26,27,28 this study found an increased risk of cataract formation with increased corticosteroid use. The risk of cataract with systemic, topical ophthalmic, or periocular corticosteroids is well established, but here we characterize the risk associated with different levels of corticosteroid therapy specifically in the intermediate uveitis context. We found an increased incidence of cataract at topical corticosteroid doses of two or more drops of corticosteroid (nearly always prednisolone acetate 1% or else equipotent doses of other topical corticosteroids) daily, as well as after four or more periocular corticosteroid injections (adjusted for the presence of anterior along with intermediate uveitis). There also tended to be higher risk with two or three prior periocular corticosteroid injections but not to a statistically significant degree. Given the 90% bilaterality of intermediate uveitis in our cohort, typically managed systemically, our results may underestimate how frequent cataract would have been if aggressive local therapy had been used, as some clinicians might do for unilateral or selected bilateral cases. Use of systemic corticosteroids was not associated with significantly increased cataract risk, although risk tended to be higher at doses above recommended short to intermediate-term maintenance levels of 7.5 mg/day or less.7 The relationship of use of intraocular corticosteroids was not studied here. Based on these results, the dose of topical corticosteroids used should be kept as low as possible to minimize the cataract risk, generally less than two drops per day except when using high doses to induce control of active anterior inflammation. In many cases of intermediate uveitis without coexisting anterior uveitis, topical corticosteroids may not be useful; they should be avoided in that setting. The increasing cataract risk with multiple periocular injections suggests that such therapy should be avoided unless the benefits outweigh the risk of cataract. Risk with oral corticosteroids was less than with topical or periocular therapy, suggesting that systemic therapy (which has been found to be highly effective in preserving visual acuity in intermediate, posterior and panuveitis)7 may be a useful cataract-avoiding strategy, which is worth taking into account along with the several other tradeoffs clinicians must consider in managing uveitis. Cataract avoidance is a secondary goal after the primary goal of inflammatory control to prevent potential permanent vision loss and ocular damage.
The significance of cataract in a uveitic eye should not be underestimated. Although cataract surgery is highly effective, it is more complex and prone to complication in eyes with a history of uveitis than in eyes with age-related cataract.2,4,29,30 Surgery should be approached with appropriate perioperative treatment, inflammation control, and carried out by an experienced specialist surgeon. While the outcomes for cataract surgery in uveitic eyes can be excellent, the procedure still carries risks and younger patients lose accommodation.4,8 Because intermediate uveitis tends to affect a younger patient population, the risk of cataract formation in this population is of more clinical significance than cataracts occurring amongst older patients.
The strengths of this study include its large sample size, and the ability to adjust for the presence or absence of anterior along with intermediate uveitis. Although all patient data were entered into a standardized database following a common protocol, the information gathered was retrospective and therefore has inherent limitations. Similarly, while a uniform definition of incident cataract was used for this study, the attribution of decreased vision to cataract relied on historical record, and the decision to undergo cataract surgery was at the discretion of individual patients and providers; there may be unknown confounding variables affecting these results. All patients were seen at tertiary care centers, so it is expected that they had more severe manifestations of disease and were more difficult to control than intermediate uveitis cases a general ophthalmologist would see (on average). However, the results still should be generalizable to tertiary care centers, and predictive factors should be generalizable unless a case could be made that the factor would affect risk differently in mild and severe cases. Additionally, as use of immunomodulatory therapies and alternate forms of corticosteroid administration are becoming more common, there may be a difference in cataract formation observed amongst patients with intermediate uveitis treated now than in the past. However, the clinics were selected in part for being early adopters of immunomodulatory therapies and immunosuppressive modalities in current use were used frequently during the study period. Also, direct assessment of/adjustment for use of corticosteroids likely addresses this issue, such that these results should be useful for predicting risk and outcomes in future patients with intermediate uveitis. While the overall sample size was large, for some covariates of interest there were limited observations with accordingly less statistical power; the precision for each variable is indicated by confidence intervals.
In conclusion, this study finds that the absolute risk of cataract formation in intermediate uveitis cases receiving tertiary uveitis care is moderate, contributing to (usually) temporary visual loss in this population, and permanent loss of accommodation in younger patients. There was an increased incidence of cataract in patients with concurrent anterior uveitis causing posterior synechiae, and in cases with epiretinal membrane. The time-updated degree or duration of intraocular inflammation in intermediate uveitis cases seemed to have less impact than was captured by inflammatory complications; the latter may represent the cumulative degree of inflammatory severity. Patients treated with higher time-updated doses of topical and periocular corticosteroids were at higher risk of cataract than those treated with lower doses, whereas low corticosteroid doses (such as prednisolone acetate 1% less than two drops per day, use of a single periocular injection or oral corticosteroids (especially at 7.5 mg/day or less)) did not, on average, appear to increase the risk of cataract substantially. Therefore, cataract risk might be reduced by minimizing use of local corticosteroid therapy, within the constraints of other potential adverse effects of treatment in a particular patient’s context.
Precis for intermediate uveitis and cataract
Using the SITE Cohort Study database, incidence of cataract in eyes with intermediate uveitis was analyzed, finding 7.6% risk at one year, and 36.6% by ten years. Risk was increased in eyes with concurrent anterior uveitis, posterior synechiae, epiretinal membrane, or increased doses of corticosteroids. Risk was not increased with 7.5mg/day oral or <2 drops/day topical corticosteroids. This large study analyzes specifically eyes with intermediate uveitis, informing counseling for these patients vis-à-vis cataract formation risk.
Supplementary Material
Funding/Support
Primary support from grants R01 EY014943 and R21 EY026717 (Dr. Kempen) and 2P30EYEY001583 (University of Pennsylvania), National Eye Institute/National Institutes of Health (Bethesda, MD); Massachusetts Eye and Ear Global Surgery Program (Boston, MA), Sight for Souls (Fort Myers, FL), and Research to Prevent Blindness (New York, NY). The funding organizations had no role in the design or conduction of this research.
Financial Disclosures
James Rosenbaum: Abbvie (Consultant); Gilead (Consultant); Janssen (Consultant); Eyevensys (Consultant); UpToDate (Consultant); Pfizer (Financial Support); Novartis (Consultant); Roche (Consultant); Alcon Research Institute (Financial Support)
Grace Levy-Clarke: Abbvie (Consultant, Lecture Fees); Allergan (Grant Support); Mallinckrodt (Consultant, Grant Support); Sanofi (Grant Support, Lecture Fees)
Eric Suhler: Eyevensys (Consultant); Santen (Consultant); EyeGate (Consultant, Financial Support); Abbvie (Consultant, Financial Support); Clearside (Consultant, Financial Support); EyePoint (Consultant, Financial Support)
Jennifer Thorne: Abbvie (Consultant)
C. Stephen Foster: Aldeyra (Consultant, Grant Support); Allakos (Consultant); Bausch & Lomb (Consultant, Grant Support); Eyegate (Consultant, Grant Support, Stock); Genentech (Consultant); Novartis (Consultant, Grant Support); pSivida (Consultant, Grant Support); Aciont (Grant Support); Alcon (Grant Support, Lecture Fees); Clearside (Grant Support); Dompé (Grant Support); Mallinckrodt (Grant Support, Lecture Fees); Allergan (Lecture Fees)
John Kempen: Gilead (Consultant—DSMC Chair), Tampa Bay Uveitis Center (consultant), Betaliq (equity owner).
mmc1.docx
mmc2.docx
Biography
Dr. John H. Kempen is Professor of Ophthalmology at Harvard Medical School and Director of Epidemiology for Ophthalmology and Senior Scientist at Massachusetts Eye and Ear. His research focuses on the epidemiology of and treatment for ocular infections and inflammatory diseases. He is Principal Investigator for the Systemic Immunosuppressive Therapy for Eye Diseases (SITE) Cohort Study and Vice-Chair of the Multicenter Uveitis Steroid Treatment (MUST) Research Group. He is President of Sight for Souls, a nonprofit eyecare organization that creates self-sustaining comprehensive eye institutes in less-developed countries.
Dr. Caroline L. Minkus is Assistant Professor of Ophthalmology at the University of Minnesota. She received her medical degree from Northwestern University Feinberg School of Medicine, where she subsequently completed ophthalmology residency. She then completed a fellowship in uveitis and ocular immunology at Massachusetts Eye and Ear, followed by a second fellowship in uveitis at Northwestern University. Her research focuses on treatment of ocular inflammatory conditions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meeting Presentation: Presented at the American Uveitis Society Winter Symposium, Park City, Utah, January 2020.
References:
- 1.Tomkins-Netzer O, Talat L, Bar A, et al. : Long-Term Clinical Outcome and Causes of Vision Loss in Patients with Uveitis. Ophthalmology 2014; 121: 2387–2392. [DOI] [PubMed] [Google Scholar]
- 2.Sen HN, Abreu FM, Louis TA, Sugar EA, et al. : Cataract Surgery Outcomes in Uveitis: The Multicenter Uveitis Steroid Treatment Trial. Ophthalmology 2016; 123: 183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blum-Hareuveni T, Seguin-Greenstein S, Kramer M, Hareuveni G, et al. : Risk Factors for the Development of Cataract in Children with Uveitis. Am J Ophthalmol 2017; 177: 139–143. [DOI] [PubMed] [Google Scholar]
- 4.Jancevski M and Foster CS. Cataracts and uveitis. Curr Opin Ophthal 2010; 21:10–14. [DOI] [PubMed] [Google Scholar]
- 5.Niederer RL, Sharief L, Bar A, et al. : Predictors of Long-Term Visual Outcome in Intermediate Uveitis. Ophthalmology 2017; 124: 393–398. [DOI] [PubMed] [Google Scholar]
- 6.Jabs DA, Nussenblatt RB, Rosenbaum JT, Standardization of Uveitis Nomenclature Working Group: Standardization of uveitis nomenclature for reporting clinical data. Results of the First international Workshop. Am J Ophthalmol 2005; 140: 509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Writing Committee for the Multicenter Uveitis Steroid Treatment (MUST) Trial and Follow-up Study Research Group: Association Between Long-Lasting Intravitreous Fluocinolone Acetonide Implant vs Systemic Anti-inflammatory Therapy and Visual Acuity at 7 Years Among Patients With Intermediate, Posterior, or Panuveitis. JAMA 2017; 317: 1993–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehta S, Linton MM, Kempen JH: Outcomes of Cataract Surgery in Patients With Uveitis: A Systematic Review and Meta-analysis. Am J Ophthalmol 2014; 158: 676–692. [DOI] [PubMed] [Google Scholar]
- 9.Kempen JH, Daniel E, Gangaputra S, et al. : Methods for Identifying Long-Term Adverse Effects of Treatment in Patients with Eye Diseases: The Systemic Immunosuppressive Therapy for Eye Disease (SITE) Cohort Study. Ophthalmic Epidemiol 2008; 15: 47–55. [DOI] [PubMed] [Google Scholar]
- 10.Kempen JH, Daniel E, Dunn JP, Foster CS, et al. : Overall and cancer related mortality among patients with ocular inflammation treated with immunosuppressive drugs: retrospective cohort study. BMJ 2009; 339: b2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ness T, Boehringer D, Heinzelmann S: Intermediate uveitis: pattern of etiology, complications, treatment and outcome in a tertiary academic center. Orphanet J Rare Dis 2017; 12: 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engelhard SB, Patel V, Reddy AK: Intermediate uveitis, posterior uveitis, and panuveitis in the Mid-Atlantic USA. Clin Ophthalmol 2015; 9: 1549–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Congdon N, O’Colmain B, Klaver CCW, Klein R, et al. : Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol 2004; 122: 477–485. [DOI] [PubMed] [Google Scholar]
- 14.Kempen JH, Van Natta ML, Altaweel MM, et al. : Factors Predicting Visual Acuity Outcome in Intermediate, Posterior, and Panuveitis: The Multicenter Uveitis Steroid Treatment (MUST) Trial. Am J Ophthalmol 2015; 160: 1133–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thorne JE, Woreta FA, Dunn JP, Jabs DA: Risk of Cataract Development among Children with Juvenile Idiopathic Arthritis-Related Uveitis Treated with Topical Corticosteroids. Ophthalmology 2010; 117: 1436–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thorne JE, Woreta F, Kedhar SR, et al. : Juvenile idiopathic arthritis-associated uveitis: incidence of ocular complications and visual acuity loss. Am J Ophthalmol 2007; 143: 840–846. [DOI] [PubMed] [Google Scholar]
- 17.Edelsten C, Lee V, Bentley CR, et al. : An evaluation of baseline risk factors predicting severity in juvenile idiopathic arthritis associated uveitis and other chronic anterior uveitis in early childhood. Br J Ophthalmol 2002; 86: 51–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ozdal PC, Berker N, Tugal-Tutkun I. Pars Planitis: Epidemiology, Clinical Characteristics, Management and Visual Prognosis. J Ophthalmic Vis Res 2015; 10: 469–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanawade R, Tsierkezou L, Bindra M, Patton N, Jones N: Visual outcomes of pars plana vitrectomy with epiretinal membrane peel in patients with uveitis. Retina 2015; 35: 736–741. [DOI] [PubMed] [Google Scholar]
- 20.Pistilli M, Joffe MM, Gangaputra SS, et al. : Contemporaneous Risk Factors for Visual Acuity in Non-Infectious Uveitis. Ocul Immunol Inflamm 2021; in press. [DOI] [PubMed] [Google Scholar]
- 21.Donaldson MJ, Pulido JS, Herman DC, Diehl N, Hodge D: Pars Planitis: A 20-Year Study of Incidence, Clinical Features, and Outcomes. Am J Ophthalmol 2007; 144: 812–817. [DOI] [PubMed] [Google Scholar]
- 22.Jabs DA, Rosenbaum JT, Foster CS, Holland GN et al. : Guidelines for the use of immunosuppressive drugs in patients with ocular inflammatory disorders: recommendations of an expert panel. Am J Ophthalmol 2000; 130: 492–513. [DOI] [PubMed] [Google Scholar]
- 23.Gicuhi S, Vedula SS, Hawkins BS, Do DV: Surgery for postvitrectomy cataract. Cochrane Database Syst Rev 2018; 2018: CD006366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kempen JH, Gewaily DY, Newcomb CW, Lisegang TL, et al. : Remission of Intermediate Uveitis: Incidence and Predictive Factors. Am J Ophthalmol 2016; 164: 110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galor A, Margolis R, Brasil OMF, Perez VL: Adverse Events after Intravitreal Triamcinolone in Patients with and without Uveitis. Ophthalmology 2007; 114: 1912–1918. [DOI] [PubMed] [Google Scholar]
- 26.Byun YS, Park Y: Complications and Safety Profile of Posterior Subtenon Injection of Triamcinolone Acdetonide. J Ocul Pharm Thera 2009; 25: 159–162. [DOI] [PubMed] [Google Scholar]
- 27.Brady CJ, Villanti AC, Law HA, Rahimy E, et al. : Corticosteroid implants for chronic non-infectious uveitis. Cochrane Database Syst Rev 2016; 2016: CD010469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sen HN, Vitale S, Gangaputra SS, Nussenblatt RB, et al. : Periocular Corticosteroid Injections in Uveitis: Effects and Complications. Ophthalmology 2014; 121: 2275–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rahman I, Jones NP: Long-term results of cataract extraction with intraocular lens implantation in patients with uveitis. Eye 2005; 19: 191–197. [DOI] [PubMed] [Google Scholar]
- 30.Chu CJ, Dick AD, Johnston RL, Yang YC, et al. : Cataract surgery in uveitis: a multicenter database study. Br J Ophthalmol 2017; 101: 1132–1137. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


