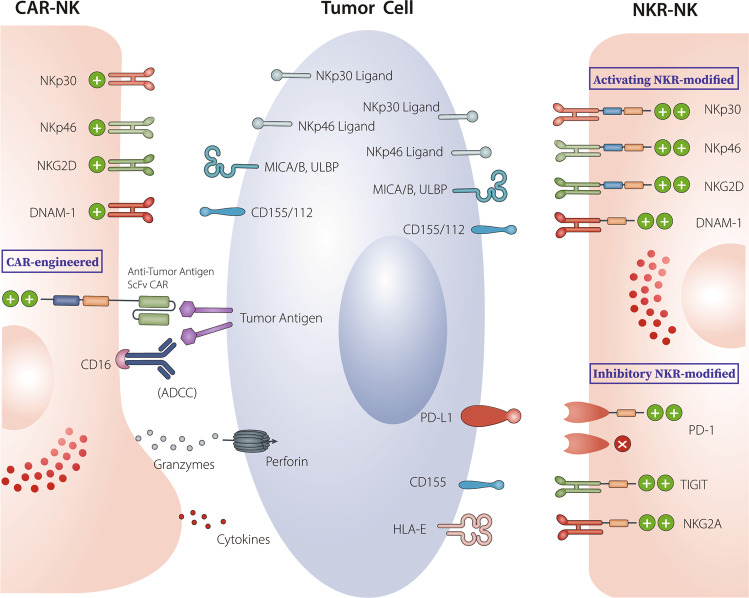
Fig. 1.
Through their extracellular scFv domain, CAR- and NKR-engineered NK cells. CAR-engineered NK cells specifically target TAAs expressed on tumor cells. They exert cytolytic activity against tumor cells in both a CAR-dependent and CAR-independent (through NKR recognition of NKR ligands and through CD16-mediated ADCC effect) manner. NKR-engineered NK cells can target a wide spectrum of tumor types with high expression of NKR ligands through NKRs. Activated receptor-modified NKR-NK cells (such as NKG2D-, NKp30- or DNAM-1-engineered NK cells) exhibit direct cytotoxicity against tumors with high expression of NKR ligands. Inhibitory NKR-modified NK cells can target tumors that express high levels of corresponding inhibitory ligands such as PD-L1. Modification with a truncated DN inhibitory receptor (e.g., DN PD-1), lacking its intracellular signaling domains, can resist checkpoint receptor-induced cell exhaustion. Inhibitory switch receptors containing an extracellular domain of inhibitory receptors (e.g., PD-1) and intracellular activating costimulatory domains (e.g., CD28) in engineered NK cells enable checkpoint receptor-mediated inhibitory signals to be switched to activating signals, thus reversing TME-mediated immunosuppression. The potential of inhibitory switch receptors, including PD-1, TIGIT, and NKG2A, used alone or in combination with other strategies for the treatment of solid tumors is under investigation