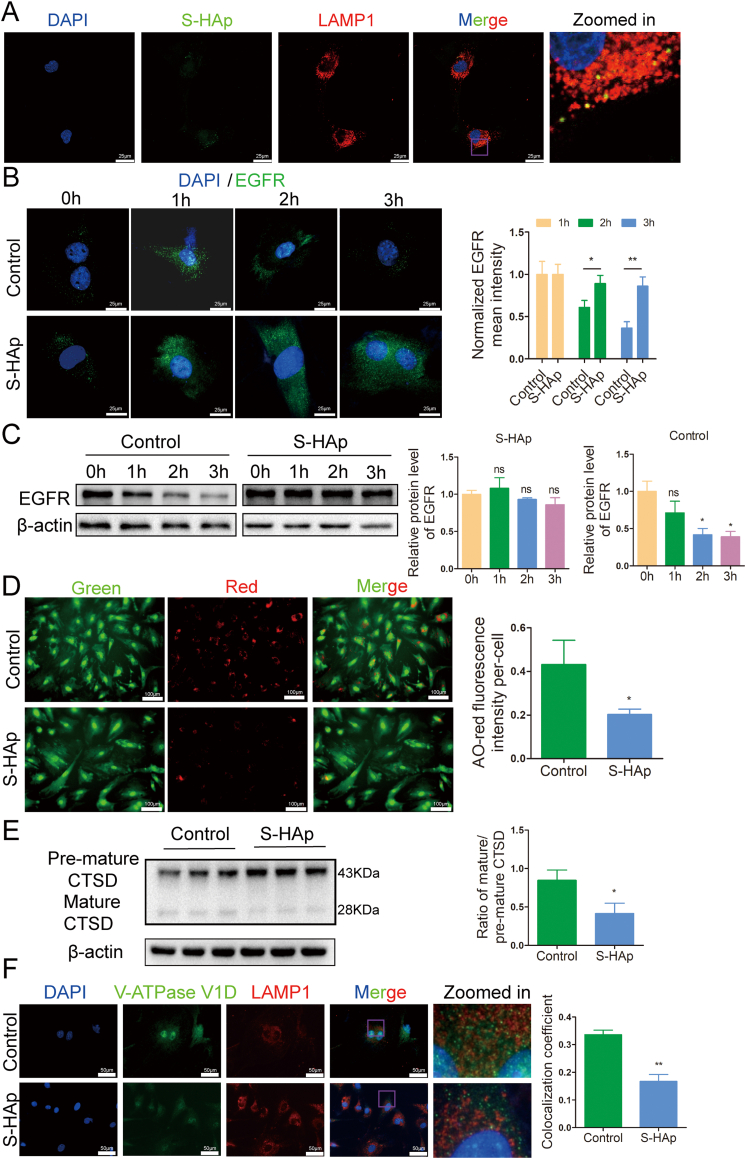
Fig. 7.
Nano-HAp impaired lysosomal degradative capacity and inhibited lysosomal acidification. A) Representative confocal immunofluorescence images show colocalization of S-HAp and LAMP1 in VSMCs. The cells were treated with 100 μg/mL fluorexon labeled S-HAp for 24 h, and then stained with LAMP1 antibody. The yellow dots represent the colocalization of S-HAp and the lysosome. B) VSMCs were treated with 100 μg/mL of S-HAp for 24 h, and then treated with 50 ng/mL EGF for 0, 1, 2 and 3 h which was removed after the specified time. The cells were fixed and immunostained with anti-EGFR antibody. Scale bars: 25 μm. Quantitative analysis of EGFR was performed by normalization to the green fluorescence intensity of cells with EGF treatment for 1 h. C) Western blot analysis of EGFR protein in the cells treated as in (A) and their quantification. D) VSMCs treated with or without S-HAp were labeled with acridine orange (AO). Red fluorescence of AO staining per cell was quantified. Scale bar: 100 μm. E) Western blot analysis of CTSD protein in VSMCs which had been treated with 100 μg/mL nHAp for 24 h. The ratio of mature-CTSD to premature-CTSD was quantified in the bar graph. F) Representative immunofluorescence images show V-ATPase V1D and LAMP1 in VSMCs which were treated with or without 100 μg/mL S-HAp for 24 h, and then stained with antibodies against V-ATPase V1D and LAMP1. The colocalization coefficients of V-ATPase V1D and LAMP1 were quantified as percentage of punctate signals of V-ATPase V1D that were positive for LAMP1. Data are presented as mean ± SD (*p < 0.05).