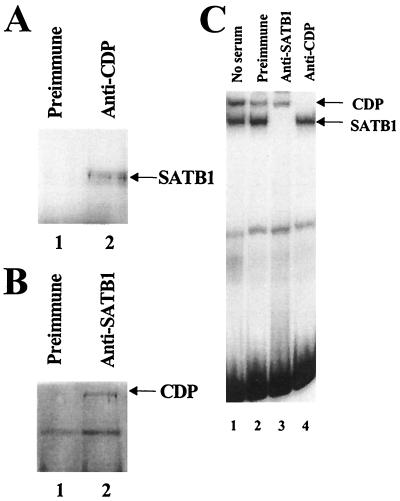
FIG. 4.
SATB1 and CDP interact in vivo. (A) Coimmunoprecipitation of SATB1 with CDP-specific antibody. Jurkat T-cell lysate was incubated with an insoluble Staphylococcus aureus suspension to eliminate nonspecific binding prior to being immunoprecipitated with preimmune (lane 1) or anti-CDP (lane 2) serum. Immunoprecipitates were resolved on an SDS–8% polyacrylamide gel, transferred to nitrocellulose, and incubated with SATB1-specific serum, followed by development as suggested by the instructions with the Amersham ECL kit. (B) Coimmunoprecipitation of CDP with SATB1-specific antibody. Western blotting was performed as described for panel A, except that immunoprecipitations were performed with preimmune (lane 1) or anti-SATB1 (lane 2) serum prior to incubation of nitrocellulose transfers with anti-CDP. (C) Specificity of anti-SATB1 and anti-CDP sera. Mink lung cell nuclear extracts were incubated with no serum (lane 1), preimmune serum (lane 2), anti-SATB1 serum (lane 3), or anti-CDP serum (lane 4), followed by a gel shift assay with the 120-bp MMTV promoter-proximal NRE probe (32) (Fig. 1A). Reactions were analyzed on a 4% nondenaturing polyacrylamide gel. The detection of both SATB1 and CDP DNA-binding activities in mink lung extracts may indicate that certain modified forms of these proteins do not interact.