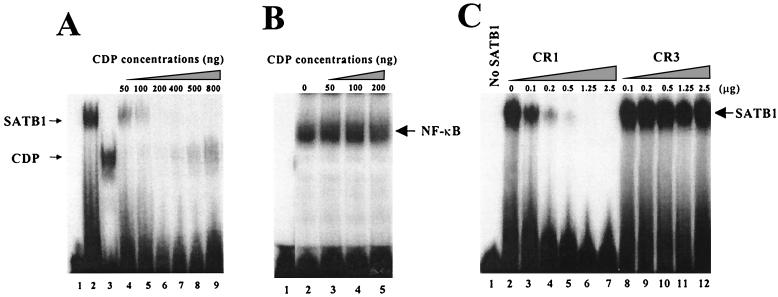
FIG. 6.
Interaction of SATB1 and CDP interferes with the DNA-binding ability of both proteins. (A) Inhibition of DNA binding after SATB1-CDP association. N-terminally truncated SATB1 and CDP were purified from recombinant fusion proteins and used for gel shift assays with the 22-bp proximal NRE probe (32) prior to electrophoresis on 4% nondenaturing polyacrylamide gels. SATB1 (500 ng, or 5.6 pmol) (lane 2) was mixed with 50 to 800 ng (0.7 to 11 pmol) (lanes 4 to 9) of CDP before addition of the labeled probe. Lane 1 shows results achieved with no added protein, whereas lane 3 shows results for 50 ng of CDP alone. The estimated molecular masses of SATB1 and CDP were 90 and 70 kDa, respectively. The 22-bp NRE probe appears to have a single binding site for CDP and for SATB1 (59). A 12-h exposure of the autoradiogram is shown. (B) NF-κB DNA binding is unaffected by CDP. LBB.A B-cell (23) nuclear extracts (4 μg) were incubated with no CDP (lane 2) or 50 to 200 ng (0.7 to 2.8 pmol) of recombinant purified CDP (lanes 3 to 5) prior to addition of the labeled NF-κB probe from the interleukin-2 receptor promoter (32) and analysis as described above. (C) The CR1, but not CR3, domain of CDP interferes with SATB1 binding to DNA. Increasing amounts of purified CR1 (9 to 225 pmol) (lanes 2 to 7) or CR3 (5.6 to 140 pmol) (lanes 8 to 12) were added to 2.5 μg (28 pmol) of purified SATB1 and incubated with the 120-bp MMTV proximal NRE probe (32) prior to analysis on a native polyacrylamide gel. Lane 1 contains no recombinant protein. Because the individual CDP cut domains do not bind well to the 120-bp probe relative to SATB1 or CDP containing the homeodomain [CDP(CR2-Cterm)], binding of CR1 and CR3 alone is not detectable under these conditions (not shown). The calculated molecular masses of the purified CR1 and CR3 proteins were approximately 11 and 18 kDa, respectively. A 2-h exposure of the autoradiogram is shown.