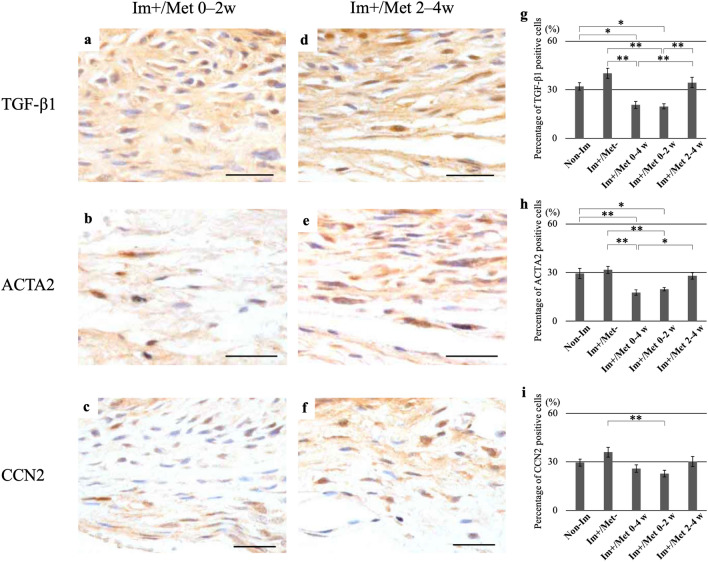
Figure 7.
Immunohistochemical analysis for the effective time of metformin administration for joint capsular fibrosis. Immunohistochemical staining for TGF-β1, ACTA2, and CCN2 in the posterior capsule. Microscopic images of specimens immunostained for TGF-β1, ACTA2, and CCN2 with DAB and counterstained with hematoxylin in the (Im+/Met 0–2 weeks) group (a–c); and (Im+/Met 2–4 weeks) group (d–f) (Scale bar: 50 μm). Percentage of TGF-β1-positive cells (g), ACTA2-positive cells (h), and CCN2-positive cells (i) to the total number of cells in the posterior joint capsule. Data are expressed as the mean ± standard errors. *p < 0.05, **p < 0.01; n = 5. TGF-β1 transforming growth factor beta 1, ACTA2 actin alpha 2, CCN2 cellular communication network factor 2, Non-Im mice with non-immobilized knees, Im+/Met− mice with immobilized knees but no metformin, Im+/Met 0–4 weeks mice with immobilized knees and metformin administered from 0 to 4 weeks, Im+/Met 0–2 weeks mice with immobilized knees and metformin administered from 0 to 2 weeks, Im+/Met 2–4 weeks mice with immobilized knees and metformin administered from 2 to 4 weeks, DAB 3,3′-diaminobenzidine.