Abstract
Exposure to maltreatment during childhood is associated with structural changes throughout the brain. However, the structural differences that are most strongly associated with maltreatment remain unclear given the limited number of whole-brain studies. The present study used machine learning to identify if and how brain structure distinguished young adults with and without a history of maltreatment. Young adults (ages 18–21, n = 384) completed an assessment of childhood trauma exposure and a structural MRI as part of the IMAGEN study. Elastic net regularized regression was used to identify the structural features that identified those with a history of maltreatment. A generalizable model that included 7 cortical thicknesses, 15 surface areas, and 5 subcortical volumes was identified (area under the receiver operating characteristic curve = 0.71, p < 0.001). Those with a maltreatment history had reduced surface areas and cortical thicknesses primarily in fronto-temporal regions. This group also had larger cortical thicknesses in occipital regions and surface areas in frontal regions. The results suggest childhood maltreatment is associated with multiple measures of structure throughout the brain. The use of a large sample without exposure to adulthood trauma provides further evidence for the unique contribution of childhood trauma to brain structure. The identified regions overlapped with regions associated with psychopathology in adults with maltreatment histories, which offers insights as to how these disorders manifest.
Subject terms: Stress and resilience, Human behaviour, Trauma, Diagnostic markers
Introduction
Young adults with a history of maltreatment, including physical and sexual abuse or neglect, during childhood are at increased risk for numerous mental health disorders including posttraumatic stress disorder and major depressive disorder [1]. This risk, which is posited to account for nearly half of all childhood mental health disorders [2], is associated with structural brain alterations. However, the association between structural differences and child maltreatment, as opposed to other forms of adversity such as economic uncertainty, is unclear [3, 4]. Thus, understanding specific associations between maltreatment and structural differences in the brain has the potential to inform brain-based models of maltreatment-related vulnerability for psychopathology.
Exposure to maltreatment during childhood is posited to affect regions involved in the detection of threatening information, the modulation of the associated stress responses, and areas of executive functioning [5]. Several studies that have examined specific associations between brain structure and maltreatment have been ROI-based studies focused to fronto-temporal regions and had small (total n < 75) sample sizes. In one study of adolescents, those with a history of abuse had reduced cortical thickness in the bilateral ventromedial prefrontal cortex, parahippocampal gyrus, inferior frontal gyrus (IFG), left temporal pole, and right middle and superior temporal gyrus [6]. Exposure to other forms of maltreatment among this sample was not reported. Another study that focused on these regions reported only a difference of reduced volume of the anterior cingulate cortex (ACC) in a sample of adolescents with a history of childhood sexual abuse [7]. However, a follow-up study did not detect any differences in the same set of ROI’s in a sample of young adults with and without a history of sexual assault using measures of cortical thickness, surface area, and subcortical volume [8]. These results provide partial support for the hypothesis that exposure to maltreatment in childhood is associated with structural differences in the fronto-temporal regions associated with stress responses. However, it is unclear how maltreatment is associated with alterations to brain structure outside of these regions.
Three studies examined structural differences across the whole brain among those with and without histories of maltreatment. The first examined differences in cortical thickness in women with maltreatment histories found thinning in somatosensory cortex, parahippocampal gyrus, precuneus, ACC, and posterior cingulate (PCC) cortex [9]. Lim et al. [10] examined differences in cortical thickness, volume, and surface area in adolescents with and without histories of maltreatment using both an ROI-based analysis of fronto-temporal regions and a whole-brain comparison. Within the ROI-based analysis, those exposed to maltreatment showed reduced cortical thickness in the right orbitofrontal cortex (OFC) and insula. In the whole-brain analysis, those with a maltreatment history also showed reduced cortical thickness in the precentral and postcentral gyri and increased cortical volume in the left and middle temporal gyri. The third examined differences across the brain in 256 adults (ages 18–35) and reported that women with a history of maltreatment had smaller volumes in the precuneus [11]. These studies suggest that maltreatment may be associated with differences beyond regions most closely associated with the response to threat.
Despite the potential benefit from whole-brain-based comparisons, there are several methodological challenges to conducting such analyses. Comparisons across the brain, at the ROI or voxel level, require more statistical tests relative to targeted ROI analyses. The increase in testing can obscure the detection of small, but clinically relevant differences [12]. The difficulty in detecting such effects is especially challenging given the modest samples (n’s < 80) used in prior work [13]. Larger samples obtained from consortium studies can address these limitations by recruiting representative samples with a spectrum of maltreatment and leverage novel analytic approaches, such as machine learning [14–16].
When examining differences across the brain, machine learning can overcome many of the limitations of more traditional comparisons [15]. Through feature selection and cross-validation, machine learning methods have shown promise in quantifying the relation between multiple brain regions and exposure to maltreatment during childhood [14, 16]. A recent study conducted used the elastic net, a supervised machine learning method, to identify the relation between structural volumes across the whole brain and overall childhood maltreatment in a sample of adults [14]. Frontal and temporal regions that were identified in prior work were most strongly associated with maltreatment histories. Additional regions also emerged including the transverse temporal region, pallidum, cerebellum, and portions of the insula. A second study that used multivariate pattern analysis to examine similar relations in a large sample of adults also showed structural associations between maltreatment and reduced volumes in frontal areas [16]. Areas in the occipital and parietal region were also identified, including the cuneus and somatosensory cortex. Of interest in both of these studies was the fact that some brain volumes were larger in maltreated compared to non-maltreated individuals, especially in the occipital cortex. The majority of prior work using univariate comparisons has primarily identified smaller regions in those with maltreatment histories. This set of findings highlights the distributed nature of the association between maltreatment and brain structure and the benefit of using methods capable of accounting for brain-wide variance.
The present study used machine learning to build a brain-based classifier to discriminate among young adults (ages 18–21) with and without a history of maltreatment. The restricted age range addressed the potential confounding effects of aging and adult trauma exposure on brain structure. Furthermore, we employed a cross-validation approach so as to determine how well the classifier performed on data that did not contribute to its creation, thereby quantifying the generalizability of the brain-based classifier. It was hypothesized that differences in fronto-temporal regions governing emotional reactivity, such as the OFC, amygdala, and hippocampus, would be most relevant in classifying those who experienced maltreatment. Based on prior work, it was also hypothesized that parietal regions, including the inferior parietal cortex (IPC), as well as the precuneus, and ACC and PCC would contribute to the classification [17]. Three measures of brain structure (surface-based thickness, surface-based areas, and subcortical volumes) were used.
Methods and materials
Participants and sample selection
Data for the current study were obtained from Wave 2 of the IMAGEN study (n = 384; age = 19.1), a large consortium study conducted across Europe. Recruitment for the IMAGEN study targeted adolescents for whom all four grandparents were the same nationality as the participant (i.e., participants were required to have four Western European grandparents); as such, the sample is racially and ethnically homogenous. Wave 2 was selected because it included an assessment of childhood maltreatment, the Childhood Trauma Questionnaire (CTQ). The maltreatment group (n = 96) scored above the established cutoffs (≥8) on the Physical Abuse or Sexual Abuse subscales of the CTQ as these scales are more closely associated with adverse outcomes [18, 19]. A portion of these individuals also presented with comorbid neglect with n = 35 and n = 20 scoring above the cutoffs for physical neglect (≥8) and emotional neglect (≥15) subscales, respectively. The comparison group (n = 288) were those who had an overall CTQ score < 28, which suggested minimal maltreatment exposure. Participants in the comparison group were age and sex matched to those in the maltreatment groups with a 3:1 (comparison:maltreatment) ratio. Demographic information is available in Table 1.
Table 1.
Demographic characteristics of the sample.
| Maltreatment (n = 96) | Comparison (n = 288) | p | |
|---|---|---|---|
| Age | 19.20 (0.79) | 19.04 (0.71) | 0.09 |
| Female sex | 32 (33.1%) | 96 (33.1%) | 1.00 |
| CTQ total score | 46.03 (13.80) | 25.90 (0.83) | <0.001 |
| SDQ score | 12.14 (5.77) | 8.22 (4.21) | <0.001 |
| WISC-IV:VC | 48.03 (9.48) | 49.78 (8.02) | 0.108 |
| SES | 17.61 (4.20) | 18.63 (3.55) | 0.038 |
p values for continuous variables (age, CTQ scores, WISC-IV, SES) are results from t-tests. For dichotomous variables (female sex), p value is obtained from a χ2.
CTQ Childhood Trauma Questionnaire, SDQ Strength and Difficulties Questionnaire, WISC-IV:VC Weschler Intelligence Scale for Children, Verbal Comprehension Subscale, SES socioeconomic status.
Measures
Childhood Trauma Questionnaire (CTQ; [20])
The CTQ is a 28-item self-report scale that assesses five categories of negative childhood experiences: emotional neglect, emotional abuse, physical neglect, physical abuse, and sexual abuse and has excellent psychometric properties [20, 21]. Each subscale is assessed with five items with scores ranging from 5 to 25 with higher scores indicating greater maltreatment.
Strengths and Difficulties Questionnaire (SDQ; [22])
The SDQ is a 25-item self-report scale that measures psychopathology across four domains: emotional, conduct, hyperactivity–inattention, and peer problems. Total difficulties scores (0–40) are calculated by summing the psychopathology domains.
Weschler Intelligence Scale for Children—IV (WISC-IV; [23])
The WISC-IV is a broad assessment of cognitive functioning. For the current study, the vocabulary subscale obtained from the first wave of the study was used as a measure of cognitive functioning. This measure was not obtained at the second wave, when the MRI data for the present analyses were obtained.
Scanning parameters
The scanning methodology for the IMAGEN study is described in Schumann et al. [24] and is available in the Standard Operating Procedures (https://imagen-europe.com/). Imaging assessments and scanning parameters were standardized across sites to ensure comparable data across locations. Data were obtained from Structural MRIs. High-resolution anatomical MRIs were obtained with a 3D T1-weighted magnetization prepared echo gradient sequence based on the Alzheimer’s Disease Neuroimaging Initiative protocol [24]. ROI measures were generated using the automated Freesurfer pipeline (version 5.3.0) (http://surfer.nmr.mgh.harvard.edu/) that used automatic labeling according to the Desikan–Killany–Tourville (DKT) atlas for cortical and subcortical regions [25]. The DKT atlas segments the cortex into 68 regions and subcortical regions into 20 ROIs. The components used in the current study included measures of cortical thickness, surface area, and subcortical volumes.
Data analytic plan
A complete overview of the data analytic plan is presented in the Supplementary materials. The primary goal of the current analysis was to identify the ROIs that best differentiated those with a history of maltreatment from those without such a history. The primary variables of interest were cortical thickness and surface area for each cortical ROI, and volume for each subcortical ROI. Sociodemographic variables were also included in the feature set: age, sex, scanning location, SES, psychopathology defined by total score on the SDQ, and the WISC-IV verbal comprehension score as a measure of cognitive functioning. All participants had complete imaging data.
Machine learning comparison
To reduce the risk of overfitting and increase the generalizability of the results, three machine learning algorithms were examined: support vector machines with a radial kernel, boosted random forests, and the elastic net. More information about the fitting procedures of each algorithm is available in the Supplementary materials (Fig. S2). The Elastic Net performed best and is described in greater detail here.
Elastic net
Models were fit with elastic net regularization (elastic net). Nested cross-validation determined the hyperparameters λ and α (Fig. S1). The elastic net is a form of regularized regression in which the goal is to obtain a parsimonious model from a large set of predictors. The elastic net was used within a nested cross-validation framework using five outer folds and ten inner folds. The pipeline as described in the Supplementary materials was repeated 100 times resulting in 500 models. Model performance was evaluated by taking the mean outcome statistic across all 500 models. For the classification analysis, the outcome statistic was the area under the receiver operating characteristic curve (AUC). Model results were validated with two methods of permutation testing: (1) permuted combinations of the dependent variable and (2) variable combinations. Variable extraction was conducted in a manner similar to Clausen et al. [14]. The variables most important to classification were identified by obtaining the mean standardized coefficient and SE for each predictor across the 500 models. Variables with coefficient means ± 2SE that did not include zero were retained. The sample size used in these calculations was 500 with zeros substituted for models in which a given feature was not selected. This approach penalizes features that are dominant in fewer models and rewards features that have reliable performance across many models. The variables identified as important were then used in a final linear regression that included only those with a maltreatment history to determine which regions of the brain scaled with the severity of maltreatment. To account for the number of variables included in this regression, an FDR correction was applied.
Results
Information about the sample is presented in Table 1. As expected, the comparison group endorsed substantially less maltreatment. There were no significant differences in the ages or sex ratio of the two groups, suggesting that the matching procedure created nearly identical groups with respect to demographics. There was no significant difference in WISC scores, t (143) = 1.62, p = 0.11. The maltreatment group reported higher psychopathology as indicated by the SDQ, t (130) = 6.16, p < 0.001. The maltreatment group reported lower SES, t (136) = 2.10, p = 0.038. All of these variables were included as features for the elastic net.
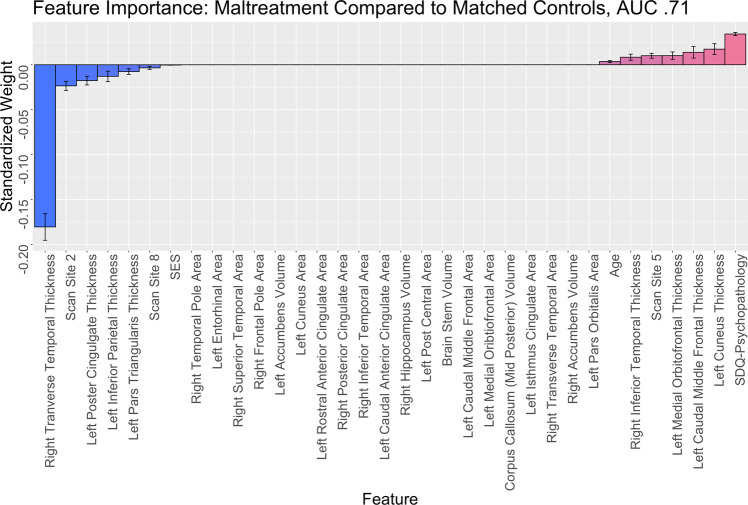
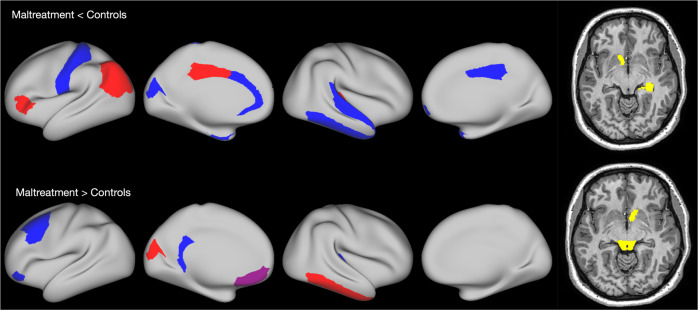
The elastic net resulted in good classification accuracy, with a mean AUC = 0.71, (SD = 0.01, range = 0.68–0.72). Permutation testing revealed that this model was highly statistically significant, (p < 0.001) (Figs. S3 and S4). The features identified spanned each structural domain: 7 cortical thickness ROI’s, 15 cortical surface area ROI’s, and 5 subcortical volume ROI’s (Table 2 and Fig. 1). The cortical thickness regions that were smaller in those with a history of maltreatment were the left IPC, the left IFG (DKT: pars triangularis), the left posterior cingulate cortex (PCC), and the right transverse temporal cortex. The cortical thickness regions that were larger in those with a history of maltreatment were the left cuneus, left caudal middle frontal cortex, left medial OFC, and right inferior temporal cortex (Fig. 2). The surface area regions that were smaller in those with maltreatment histories were the left dorsal (dACC; DKT: caudal anterior cingulate) and rostral anterior cingulate cortex (rACC), left cuneus, left entorhinal, left postcentral cortex, right inferior temporal, right superior temporal, right frontal pole, and right temporal pole. The surface area regions that were larger in those with maltreatment histories were the left caudal middle frontal region, left isthmus cingulate, left medial OFC, left IFG (DKT: pars orbitalis), and right transverse temporal cortex. The subcortical regions that were smaller among those with a maltreatment history were the left accumbens and right hippocampus. Those with maltreatment histories had larger brain stem volumes, right accumbens volumes, and the mid posterior of the corpus callosum. Six sociodemographic features were also identified: age, SES, psychopathology, and three location sites.
Table 2.
Variables contributing to the classification of childhood maltreatment.
| Direction of difference | |
|---|---|
| Regions in the left hemisphere | |
| Accumbens volume | Maltreatment < control |
| Caudal anterior cingulate area | Maltreatment < control |
| Rostral anterior cingulate area | Maltreatment < control |
| Cuneus area | Maltreatment < control |
| Entorhinal area | Maltreatment < control |
| Left postcentral area | Maltreatment < control |
| Inferior parietal thickness | Maltreatment < control |
| Pars triangularis thickness | Maltreatment < control |
| Posterior cingulate cortex thickness | Maltreatment < control |
| Caudal middle frontal area | Maltreatment > control |
| Isthmus cingulate area | Maltreatment > control |
| Medial orbitofrontal area | Maltreatment > control |
| Pars orbitalis area | Maltreatment > control |
| Caudal middle frontal thickness | Maltreatment > control |
| Cuneus thickness | Maltreatment > control |
| Medial orbitofrontal thickness | Maltreatment > control |
| Regions in the right hemisphere | |
| Inferior temporal area | Maltreatment < control |
| Posterior cingulate area | Maltreatment < control |
| Superior temporal area | Maltreatment < control |
| Frontal pole area | Maltreatment < control |
| Temporal pole area | Maltreatment < control |
| Transverse temporal thickness | Maltreatment < control |
| Hippocampus volume | Maltreatment < control |
| Inferior temporal thickness | Maltreatment > control |
| Transverse temporal area | Maltreatment > control |
| Accumbens volume | Maltreatment > control |
| Additional regions | |
| Brain stem volume | Maltreatment > control |
| Corpus callosum—mid posterior | Maltreatment > control |
| Covariates | |
| Age | Maltreatment > control |
| SDQ total score | Maltreatment > control |
| SES | Maltreatment < control |
| Scan site 2 | Maltreatment < control |
| Scan site 5 | Maltreatment > control |
| Scan site 8 | Maltreatment < control |
Fig. 1. Importance plot of features involved in classification.
Weights represent prediction of maltreatment group. Negative weights indicate that this region is smaller in those with histories of maltreatment. Positive weights indicate that this region is larger in those with a history of maltreatment.
Fig. 2. Brain regions involved in classification of those with a history of maltreatment.
Red regions are cortical thickness, blue regions are surface areas, purple regions are those showing both cortical thickness and surface area effects, and yellow regions are subcortical volumes.
Linear regression was then used to determine if there was a continuous relation among those regions identified by the elastic net and maltreatment severity in the maltreatment group. None of the regions were significantly associated with severity (FDR-corrected p’s > 0.22; Fig. S5). The identified regions, however, performed better as a predictor of severity of combined physical and sexual abuse than of combined physical and emotional neglect (Fig. S6).
Discussion
The results of the present study are among the first to show that multivariate structural data from the whole brain can accurately classify young adults with and without a history of maltreatment. These results were obtained in a sample that was larger than prior work and had a restricted age range. The use of a restricted age reduced the potential confounds of adulthood exposure and developmental age. The larger sample allowed for the use of repeated cross-validation, a method that increases confidence in the generalizability of the results as it estimates performance of a model on data not used in model construction [15]. These methodological improvements provide further evidence for the need to examine the whole brain to both support and expand our understanding of the possible impact of childhood maltreatment.
Those with a history of childhood maltreatment had reduced cortical thickness in the left IPC. A recent large-scale study examining the association of maltreatment and depression also showed that those with a history of maltreatment had reduced thickness in this area [26]. The IPC is posited to be a junction point between the dorsal and ventral attention networks and is thought to be key in managing the allocation of attention between external and internal stimuli [27]. Diminished cortical thickness in this region is associated with elevated distractibility and perseverating on threatening information [28]. Those with a history of maltreatment have shown hyperreactivity toward threatening information, and alterations in the IPC may be a potential mechanism [29, 30]. Indeed, prior studies have observed altered function and structure in this region in survivors of childhood maltreatment, particularly in association with attentional dysfunction [17, 31, 32].
Several regions associated with the default mode network (DMN) had reduced cortical thickness (PCC, IFG) and surface area (rACC, dACC, OFC, STG, and entorhinal) in those with a history of maltreatment [33]. Reduced functional connectivity in the DMN is a consistently reported finding among adults with a history of trauma exposure [34, 35]. However, the present study is among a handful that have also shown structural differences in these regions [36]. These regions collectively are thought to be involved in self-referential processes and emotion regulation [37]. The alterations in these regions are thought to correspond to reexperiencing symptoms that are often observed among those with a trauma history [38]. The PCC and rACC are specifically implicated in emotion regulation [37] and the regulation of arousal [39]. These regions further highlight how exposure to maltreatment specifically may be associated with regions involved in the regulation of emotion. That these findings were obtained in a sample of young adults adds to the evidence that this region is affected by childhood experiences as opposed to those that occurred in adulthood.
Several areas were identified that had not been the focus of prior work and warrant further attention. Reduced cortical thickness of the right transverse temporal areas was associated with a history of maltreatment. This region in the right hemisphere was also identified in a prior study that used the elastic net [14], however, the relation was in the opposite direction. That is, larger volumes were associated with more severe maltreatment. A potential explanation for this change in direction is the difference in ages between the sample in the current study (young adults) and the prior study (middle aged adults). This region undergoes cortical thinning over the course of adulthood, but the rate of this thinning has been associated with the presence of psychopathology in prior work [40]. The presence of this region in both studies suggests this area may be associated with maltreatment, but the directional relation of this association may change as a function age. Increased cortical thickness and surface areas in several regions were associated with a history of maltreatment including the left cuneus. A recent study using MVPA reported a larger volume in the cuneus in those with a history of maltreatment, but not those with other forms of maltreatment history [16]. These findings warrant further investigation of the association of maltreatment and cuneus thickness.
The present study had several notable strengths including a relatively large sample and the use of a sophisticated analytic strategy. It also had a number of limitations. First, maltreatment history was assessed using a single self-report measure. Future work should make efforts to more fully characterize the severity and timing of maltreatment as these are critical to understanding how maltreatment impacts the brain [41]. Second, the present study focused only on structural data. Efforts should be made to integrate other modes of imaging data to determine how structural and functional data can identify those with maltreatment histories. Third, the study relied solely on linear combinations of brain regions as opposed to interactions. It is likely that the differences in cortical thickness and surface area may interact with one another to further distinguish these groups. Fourth, the assessment of psychopathology was limited to a single measure. Given that differences throughout the brain are likely associated with the presence of psychopathology, future work should incorporate more detailed information about mental health severity. Fifth, types of child maltreatment are highly comorbid and the sample in the current analyses reported histories of both abuse and neglect. Although the findings identify associations between maltreatment and brain structure, they are unable to disentangle the unique effect of a given type of maltreatment (abuse or neglect) on brain structure. Additionally, the study sample was collected throughout Europe and chosen to be homogenous with regards to racial and ethnic identity. Therefore, it is unclear how generalizable the results of the present study may be to a more ethnic and racially diverse population. Finally, the sample size did permit the use of an independent hold-out sample for a final validation, which may impact the generalizability of the results.
The results of the present study provide further evidence for the distributed effect of maltreatment during childhood across the brain. This study also highlighted how machine learning can identify potential candidate regions for future investigation. The IPC and transverse temporal region have been identified in prior work using machine learning but have not been a primary area of study within the literature. Taken together, these findings suggest that the structure of regions involved in the perception of threatening information and emotion regulation may be the most affected by exposure to maltreatment, but the effects extend well beyond these areas.
Funding and disclosure
The authors declare no competing interests. This work received support from the following sources: NIMH K08MH107661-A1, NIMH K08MH121654-A1, the European Union-funded FP6 Integrated Project IMAGEN (Reinforcement-related behavior in normal brain function and psychopathology) (LSHM-CT- 2007-037286), the Horizon 2020 funded ERC Advanced Grant “STRATIFY” (Brain network-based stratification of reinforcement-related disorders) (695313), ERANID (Understanding the Interplay between Cultural, Biological and Subjective Factors in Drug Use Pathways) (PR-ST-0416-10004), BRIDGET (JPND: Brain Imaging, cognition Dementia and next generation Genomics) (MR/N027558/1), Human Brain Project (HBP SGA 2, 785907), the FP7 project MATRICS (603016), the Medical Research Council Grant “c-VEDA” (Consortium on Vulnerability to Externalizing Disorders and Addictions) (MR/N000390/1), the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London, the Bundesministeriumfür Bildung und Forschung (BMBF grants 01GS08152, 01EV0711; Forschungsnetz AERIAL 01EE1406A, 01EE1406B), the Deutsche Forschungsgemeinschaft (DFG grants SM 80/7-2, SFB 940/2), the Medical Research Foundation and Medical Research Council (grants MR/R00465X/1 and MR/S020306/1), the National Institutes of Health (NIH) funded ENIGMA (grants 5U54EB020403-05 and 1R56AG058854-01). Further support was provided by grants from: ANR (project AF12-NEUR0008-01—WM2NA, and ANR-12-SAMA-0004), the Fondation de France, the Fondation pour la Recherche Médicale, the Mission Interministérielle de Lutte-contre-les-Drogues-et-les-Conduites-Addictives (MILDECA), the Assistance-Publique-Hôpitaux-de-Paris and INSERM (interface grant), Paris Sud University IDEX 2012; the National Institutes of Health, Science Foundation Ireland (16/ERCD/3797), USA (Axon, Testosterone and Mental Health during Adolescence; RO1 MH085772-01A1), and by NIH Consortium grant U54 EB020403, supported by a cross-NIH alliance that funds Big Data to Knowledge Centres of Excellence.
Supplementary information
Author contributions
MP: conceptualized the study, conducted the primary analyses, and drafted the majority of the paper. MA: contributed to the conceptualization of the study, provided critical resources for interpreting the data, and assisted in drafting the paper. S. Hahn: contributed to the analyses and creating figures for the paper, and provided substantial guidance on the analysis. ACJ: provided and assisted in analyzing the data and assisted in drafting the paper. NF: contributed to the conceptualization of the study, provided critical resources for interpreting the data, and assisted in drafting the paper. ZMFB: critically revised the paper. ACL: critically revised the paper. KS-C: critically revised the paper. BC: critically revised the paper, provided analytic resources, and aided in interpreting the paper. AP: critically revised the paper and provided meaningful interpretation of the data. KP: critically revised the paper. NA: provided substantial guidance on the analysis. TB: reviewed early conceptualization of the study, reviewed the paper, and offered final approval of the version to be published. ALWB: reviewed early conceptualization of the study, reviewed the paper, and offered final approval of the version to be published. EBQ: reviewed early conceptualization of the study, reviewed the paper, and offered final approval of the version to be published. SD: reviewed early conceptualization of the study, reviewed the paper, and offered final approval of the version to be published. HF: reviewed early conceptualization of the study, reviewed the paper, and offered final approval of the version to be published. AG: reviewed early conceptualization of the study, reviewed the paper, and offered final approval of the version to be published. PG: reviewed early conceptualization of the study, reviewed the paper, and offered final approval of the version to be published. AH: reviewed early conceptualization of the study, reviewed the paper, and offered final approval of the version to be published. BI: reviewed early conceptualization of the study, reviewed the paper, and offered final approval of the version to be published. J-LM: reviewed early conceptualization of the study, reviewed the paper, and offered final approval of the version to be published. M-LP: reviewed early conceptualization of the study, reviewed the paper, and offered final approval of the version to be published. EA: reviewed early conceptualization of the study, reviewed the paper, and offered final approval of the version to be published. FN: reviewed early conceptualization of the study, reviewed the paper, and offered final approval of the version to be published. DPO: reviewed early conceptualization of the study, reviewed the paper, and offered final approval of the version to be published. LP: reviewed early conceptualization of the study, reviewed the paper, and offered final approval of the version to be published. S. Hohmann: reviewed early conceptualization of the study, reviewed the paper, and offered final approval of the version to be published. JHF: reviewed early conceptualization of the study, reviewed the paper, and offered final approval of the version to be published. MNS: reviewed early conceptualization of the study, reviewed the paper, and offered final approval of the version to be published. HW: reviewed early conceptualization of the study, reviewed the paper, and offered final approval of the version to be published. RW: reviewed early conceptualization of the study, reviewed the paper, and offered final approval of the version to be published. GS: reviewed early conceptualization of the study, reviewed the paper, and offered final approval of the version to be published. HG: reviewed early conceptualization of the study, substantially contributed to the revision of the paper and interpretation, reviewed the paper, and offered final approval of the version to be published.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41386-021-00987-7.
References
- 1.Edwards VJ, Holden GW, Felitti VJ, Anda RF. Relationship between multiple forms of childhood maltreatment and adult mental health in community respondents: results from the adverse childhood experiences study. Am J Psychiatry. 2003;160:1453–60. doi: 10.1176/appi.ajp.160.8.1453. [DOI] [PubMed] [Google Scholar]
- 2.Teicher MH, Samson JA. Annual research review: enduring neurobiological effects of childhood abuse and neglect. J Child Psychol Psychiatry. 2016;57:241–66. doi: 10.1111/jcpp.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peverill M, Dirks MA, Narvaja T, Herts KL, Comer JS, McLaughlin KA. Socioeconomic status and child psychopathology in the United States: a meta-analysis of population-based studies. Clin Psychol Rev. 2021;83:101933. doi: 10.1016/j.cpr.2020.101933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McLaughlin KA, Weissman D, Bitrán D. Childhood adversity and neural development: a systematic review. Annu Dev Dev Psychol. 2019;1:277–312. doi: 10.1146/annurev-devpsych-121318-084950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McEwen BS. Brain on stress: how the social environment gets under the skin. Proc Natl Acad Sci. 2012;109:17180–5. doi: 10.1073/pnas.1121254109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gold AL, Sheridan MA, Peverill M, Busso DS, Lambert HK, Alves S, et al. Childhood abuse and reduced cortical thickness in brain regions involved in emotional processing. J Child Psychol Psychiatry. 2016;57:1154–64. doi: 10.1111/jcpp.12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rinne-Albers MA, Pannekoek JN, van Hoof M-J, van Lang ND, Lamers-Winkelman F, Rombouts SA, et al. Anterior cingulate cortex grey matter volume abnormalities in adolescents with PTSD after childhood sexual abuse. Eur Neuropsychopharmacol. 2017;27:1163–71. doi: 10.1016/j.euroneuro.2017.08.432. [DOI] [PubMed] [Google Scholar]
- 8.Rinne-Albers MA, Boateng CP, van der Werff SJ, Lamers-Winkelman F, Rombouts SA, Vermeiren RR, et al. Preserved cortical thickness, surface area and volume in adolescents with PTSD after childhood sexual abuse. Sci Rep. 2020;10:1–9. doi: 10.1038/s41598-020-60256-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heim CM, Mayberg HS, Mletzko T, Nemeroff CB, Pruessner JC. Decreased cortical representation of genital somatosensory field after childhood sexual abuse. Am J Psychiatry. 2013;170:616–23. doi: 10.1176/appi.ajp.2013.12070950. [DOI] [PubMed] [Google Scholar]
- 10.Lim L, Hart H, Mehta M, Worker A, Simmons A, Mirza K, et al. Grey matter volume and thickness abnormalities in young people with a history of childhood abuse. Psychol Med. 2018;48:1034–46. doi: 10.1017/S0033291717002392. [DOI] [PubMed] [Google Scholar]
- 11.Everaerd D, Klumpers F, Zwiers M, Guadalupe T, Franke B, van Oostrom I, et al. Childhood abuse and deprivation are associated with distinct sex-dependent differences in brain morphology. Neuropsychopharmacology. 2016;41:1716–23. doi: 10.1038/npp.2015.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jollans L, Whelan R. Neuromarkers for mental disorders: harnessing population neuroscience. Front Psychiatry. 2018;9:242–56. doi: 10.3389/fpsyt.2018.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whelan R, Garavan H. When optimism hurts: Inflated predictions in psychiatric neuroimaging. Biol Psychiatry. 2014;75:746–8. doi: 10.1016/j.biopsych.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 14.Clausen AN, Aupperle RL, Yeh H-W, Waller D, Payne J, Kuplicki R, et al. Machine learning analysis of the relationships between gray matter volume and childhood trauma in a transdiagnostic community-based sample. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019;4:734–42. doi: 10.1016/j.bpsc.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jollans L, Boyle R, Artiges E, Banaschewski T, Desrivières S, Grigis A, et al. Quantifying performance of machine learning methods for neuroimaging data. NeuroImage. 2019;199:351–65. doi: 10.1016/j.neuroimage.2019.05.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Popovic D, Ruef A, Dwyer DB, Antonucci LA, Eder J, Sanfelici R, et al. Traces of trauma: a multivariate pattern analysis of childhood trauma, brain structure, and clinical phenotypes. Biol Psychiatry. 2020. 10.1016/j.biopsych.2020.05.020. [DOI] [PubMed]
- 17.Blair KS, Aloi J, Crum K, Meffert H, White SF, Taylor BK, et al. Association of different types of childhood maltreatment with emotional responding and response control among youths. JAMA Netw Open. 2019;2:e194604. doi: 10.1001/jamanetworkopen.2019.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walker EA, Unutzer J, Rutter C, Gelfand A, Saunders K, VonKorff M, et al. Costs of health care use by women HMO members with a history of childhood abuse and neglect. Arch Gen Psychiatry. 1999;56:609–13. doi: 10.1001/archpsyc.56.7.609. [DOI] [PubMed] [Google Scholar]
- 19.McLaughlin KA, Sheridan MA. Beyond cumulative risk: a dimensional approach to childhood adversity. Curr Dir Psychol Sci. 2016;25:239–45. doi: 10.1177/0963721416655883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bernstein F. Manual for the Childhood Trauma Questionnaire. San Antonio, TX, The Psychological Corporation 1998.
- 21.Bernstein DP, Ahuvalia T, Pogge D, Handelsman L. Validity of the Childhood Trauma Questionnaire in an Adolescent Psychiatric Population. J Am Acad Child Adolesc Psychiatry. 1997;36:340–8. doi: 10.1097/00004583-199703000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Goodman R. Psychometric properties of the Strengths and Difficulties Questionnaire. J Am Acad Child Adolesc Psychiatry. 2001;40:1337–45. doi: 10.1097/00004583-200111000-00015. [DOI] [PubMed] [Google Scholar]
- 23.Yeates KO, Donders J. WISC-IV clinical use and interpretation: Scientist-practitioner perspectives. San Diego, CA: Elsevier Academic Press; 2005. pp. 415–34. [Google Scholar]
- 24.Schumann G, Loth E, Banaschewski T, Barbot A, Barker G, Büchel C, et al. The IMAGEN study: reinforcement-related behaviour in normal brain function and psychopathology. Mol Psychiatry. 2010;15:1128–39. doi: 10.1038/mp.2010.4. [DOI] [PubMed] [Google Scholar]
- 25.Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang M-C, et al. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. NeuroImage. 2009;46:786–802. doi: 10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tozzi L, Garczarek L, Janowitz D, Stein DJ, Wittfeld K, Dobrowolny H, et al. Interactive impact of childhood maltreatment, depression, and age on cortical brain structure: mega-analytic findings from a large multi-site cohort. Psychol Med. 2020;50:1020–31. doi: 10.1017/S003329171900093X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci. 2006;103:10046–51. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 29.Gibb BE, Schofield CA, Coles ME. Reported history of childhood abuse and young adults’ information-processing biases for facial displays of emotion. Child Maltreat. 2009;14:148–56. doi: 10.1177/1077559508326358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Günther V, Dannlowski U, Kersting A, Suslow T. Associations between childhood maltreatment and emotion processing biases in major depression: results from a dot-probe task. BMC Psychiatry. 2015;15:123. doi: 10.1186/s12888-015-0501-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lim L, Hart H, Mehta MA, Simmons A, Mirza K, Rubia K. Neurofunctional abnormalities during sustained attention in severe childhood abuse. PLoS ONE. 2016;11:e0165547. doi: 10.1371/journal.pone.0165547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sheffield JM, Williams LE, Woodward ND, Heckers S. Reduced gray matter volume in psychotic disorder patients with a history of childhood sexual abuse. Schizophr Res. 2013;143:185–91. doi: 10.1016/j.schres.2012.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akiki TJ, Averill CL, Abdallah CG. A network-based neurobiological model of PTSD: evidence from structural and functional neuroimaging studies. Curr Psychiatry Rep. 2017;19:81. doi: 10.1007/s11920-017-0840-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DiGangi JA, Tadayyon A, Fitzgerald DA, Rabinak CA, Kennedy A, Klumpp H, et al. Reduced default mode network connectivity following combat trauma. Neurosci Lett. 2016;615:37–43. doi: 10.1016/j.neulet.2016.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bluhm RL, Williamson PC, Osuch EA, Frewen PA, Stevens TK, Boksman K, et al. Alterations in default network connectivity in posttraumatic stress disorder related to early-life trauma. J Psychiatry Neurosci Jpn. 2009;34:187–94. [PMC free article] [PubMed] [Google Scholar]
- 36.Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010;65:550–62. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Etkin A, Prater KE, Hoeft F, Menon V, Schatzberg AF. Failure of anterior cingulate activation and connectivity with the amygdala during implicit regulation of emotional processing in generalized anxiety disorder. Am J Psychiatry. 2010;167:545–54. doi: 10.1176/appi.ajp.2009.09070931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sripada RK, King AP, Welsh RC, Garfinkel SN, Wang X, Sripada CS, et al. Neural dysregulation in posttraumatic stress disorder: evidence for disrupted equilibrium between salience and default mode brain networks. Psychosom Med. 2012;74:904–11. doi: 10.1097/PSY.0b013e318273bf33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shin LM, Bush G, Milad MR, Lasko NB, Brohawn KH, Hughes KC, et al. Exaggerated activation of dorsal anterior cingulate cortex during cognitive interference: a monozygotic twin study of posttraumatic stress disorder. Am J Psychiatry. 2011;168:979–85. doi: 10.1176/appi.ajp.2011.09121812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zielinski BA, Prigge MBD, Nielsen JA, Froehlich AL, Abildskov TJ, Anderson JS, et al. Longitudinal changes in cortical thickness in autism and typical development. Brain. 2014;137:1799–812. doi: 10.1093/brain/awu083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Teicher MH, Parigger A. The ‘maltreatment and abuse chronology of exposure’ (MACE) scale for the retrospective assessment of abuse and neglect during development. PLoS ONE. 2015;10:e0117423. doi: 10.1371/journal.pone.0117423. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.