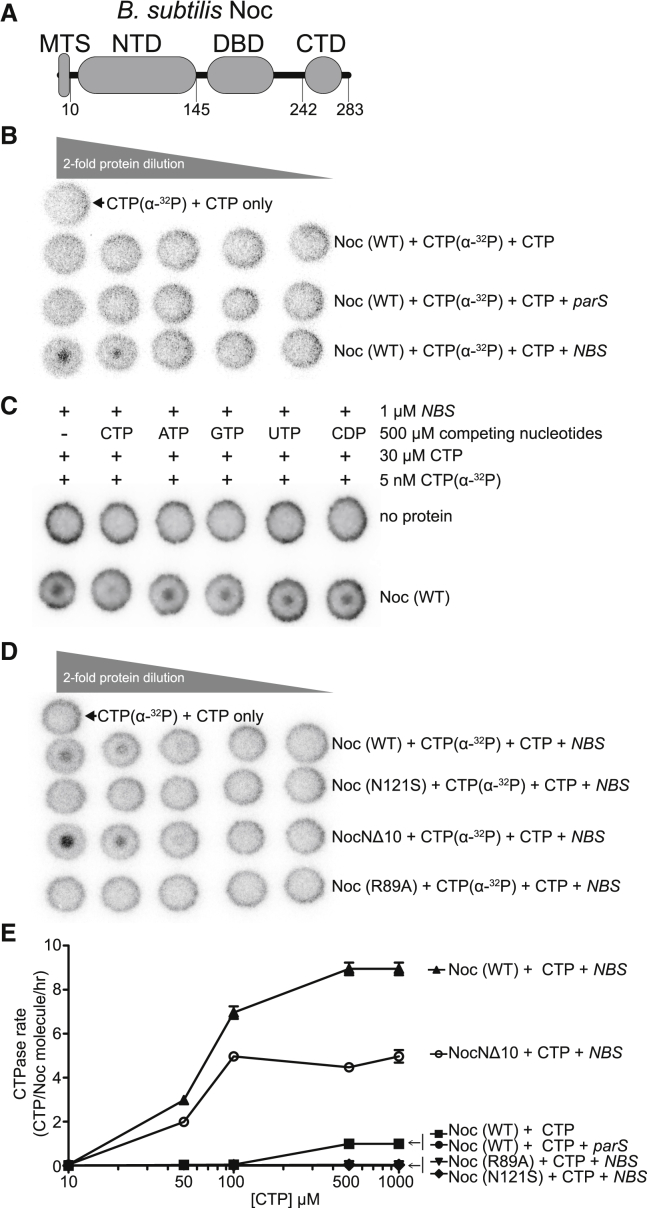
Figure 1.
Noc binds and hydrolyzes CTP in the presence of NBS DNA
(A) The domain architecture of B. subtilis Noc: a membrane-targeting sequence (MTS), an N-terminal domain (NTD), a central DNA-binding domain (DBD), and a C-terminal domain (CTD).
(B–D) CTP binding as monitored by DRaCALA assay using radiolabeled CTP α-P32. The bull’s-eye staining indicates CTP binding due to a more rapid immobilization of protein-ligand complexes compared with free ligands. The starting concentration of Noc used in all panels was 30 μM. The concentrations of CTP α-P32, unlabeled CTP, and a 22 bp parS/NBS DNA used in all panels were 5 nM, 30 μM, and 1 μM, respectively.
(E) CTP hydrolysis rates of Noc (WT) and variants were measured by continuous detection of released inorganic phosphates (see STAR Methods). CTPase rates were measured at increasing concentrations of CTP. All reactions contained 1 μM Noc (WT/variants) ± 1 μM 22 bp NBS or parS DNA and an increasing concentration of CTP (0, 10, 50, 100, 500, and 1,000 μM). Experiments were triplicated, and the SDs of the CTPase rates were presented.