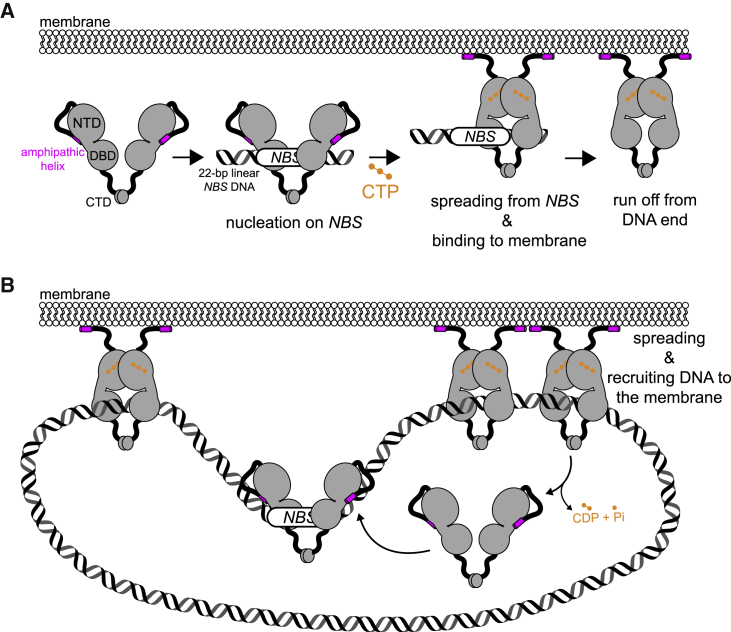
Figure 7.
A model for a CTP-dependent regulation of membrane-binding activity of Noc
(A) Noc binds specifically to NBS site to nucleate on DNA. In the apo- or NBS-associated form, the amphipathic helix (magenta) adopts an autoinhibitory conformation and thus cannot bind to the membrane. NBS-binding stimulates Noc to bind CTP (orange). Concomitantly, CTP induces a sliding clamp conformation in Noc that can run off open ends of a linear 22 bp NBS DNA. In this state, the amphipathic helix is likely liberated from the autoinhibitory conformation, thus enables Noc-CTP to bind to the membrane.
(B) When a circular NBS plasmid with no open end was used, a sliding clamp of Noc entraps plasmid DNA and recruit DNA to the membrane. In the presence of CTP, a tripartite membrane-protein-DNA linkage is formed. CTP hydrolysis is not required for membrane binding or DNA recruitment but might have a role in releasing Noc from DNA and the membrane.