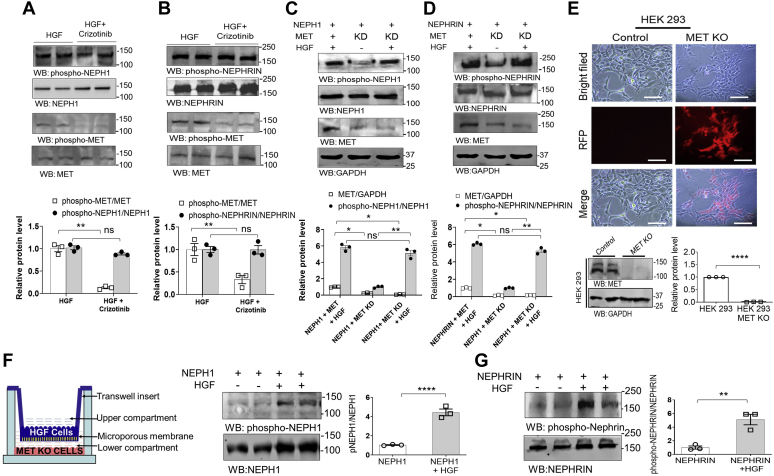
Figure 4.
The MET receptor is not required for HGF-induced NEPHRIN and NEPH1 phosphorylation. Recombinant HGF (20 ng/ml) was added to HEK293 cells overexpressing (A) NEPH1-FLAG or (B) NEPHRIN-FLAG in the presence or absence of 100 nM Crizotinib, a MET inhibitor. Phosphorylation of NEPH1 and NEPHRIN was unchanged by the presence of Crizotinib. Data are presented as mean ± SEM, and p-values were calculated using the Kruskal–Wallis one-way analysis of variance. C, D, NEPH1-FLAG or NEPHRIN-FLAG were overexpressed in HEK293 cells with stable shRNA-mediated knockdown (KD) of the MET receptor. Recombinant HGF (20 ng/ml) was added to these cells, and NEPHRIN and NEPH1 phosphorylation in the cell lysate showed no change in phosphorylation following MET knockdown (KD). Data are presented as mean ± SEM, and p-values were calculated using the Kruskal–Wallis one-way analysis of variance. E, using CRISPR-Cas9, stable MET knockout HEK293 cells were generated and transfected with NEPHRIN- and NEPH1-expressing plasmids. Red fluorescent protein (RFP) is a marker for transfection and confirms the stable knockout of MET post puromycin selection. Data are presented as mean ± SEM, and p-values were calculated using a two-tailed Student’s t test. The scale bar represents 25 μm. F and G, using HGF-overexpressing cells grown on the Transwell filter and HEK293 MET knockout cells expressing NEPH1 or NEPHRIN growing on the plastic at the bottom of well (first panel, schematic), we demonstrate that NEPH1 and NEPHRIN phosphorylation occurs following complete loss of the MET receptor. Data are presented as mean ± SEM, and p-values were calculated using a two-tailed Student’s t test. ns, nonsignificant, ∗p ≤ 0.05, ∗∗p ≤ 0.01, ∗∗∗∗p ≤ 0.0001. All experiments were performed in triplicate and repeated three times with similar results, and representative images of the results are presented in the figure.