Abstract
Objective: The aim of this investigation was to clarify the correlation of early cognitive dysfunction (CD) with inflammatory factors and metabolic indicators in patients with Alzheimer’s disease (AD). Methods: Eighty patients with AD who were referred to our hospital from May 2019 to May 2020 were selected as the research group (RG) and 71 non-AD patients served as the control group (CG). The two groups were compared regarding the changes in their mini-mental state examination (MMSE) scores and inflammatory factors as well as metabolic indicators. The correlation of MMSE with inflammatory factors and metabolic indicators was analyzed by Pearson correlation analysis. Results: The RG presented with lower MMSE scores than the CG. Interleukin (IL-6), C-reactive protein (CRP), IL-1β levels, low density lipoprotein cholesterol (LDL-C), total cholesterol (TC), triglyceride (TG), fasting plasma glucose (FPG), and systolic blood pressure (SBP) were all higher in the RG as compared to the CG, while high density lipoprotein cholesterol (HDL-C), ApoE and ApoAI were lower (all P<0.05). The MMSE score was negatively associated with IL-6, CRP, IL-1β, LDL-C, TC, TG, FPG and SBP levels, and was positively correlated with HDL-C, ApoE and ApoAI levels. Conclusions: Inflammatory factors and metabolic indicators are highly correlated with early CD in patients with AD, and thus may be excellent potential indicators for the future diagnosis and treatment of AD.
Keywords: Alzheimer’s disease, early cognitive dysfunction, inflammatory factors, metabolic indicators, correlation research
Introduction
Alzheimer’s disease (AD), the most common cause of dementia characterized by progressive cognitive dysfunction (CD) and behavioral impairment, is a central nervous system degenerative disorder mostly occurring durring senectitude and presenium [1,2]. Statistics show that AD affects 15 million people worldwide [3], and its incidence is in parallel with population aging and it is associated with the increasing prevalence of many dementia-related neurodegenerative diseases in recent years [4]. AD can cause varying degrees of memory impairment, aphasia, apraxia, agnosia, visuospatial dysfunction, abstract thinking and computing ability damage, as well as personality and behavior changes [5]. Among them, CD is the most significant and serious clinical manifestation of AD [6], which can also leads to a drastic decline of patients’ self-care ability, adversely affecting the quality of life of patients [7]. Given that there is currently no specific medicine that can effectively cure AD in the clinic, a combination of drug or non-drug therapy and long-term careful nursing is often required to relieve symptoms and delay the disease progression [8]. Clinically, it is believed that the key to AD treatment is to ameliorate the CD and improve treatment compliance of patients, thus improving their prognosis [9]. However, no research has been conducted to confirm the exact mechanism by which CD occurs in AD [10].
With the deepening of research, accumulating studies have pointed out that inflammatory factors and blood metabolism are key factors in CD caused by multiple neurological diseases [11]. Inflammatory factors can damage and destroy neurovascular and neuronal cells by inducing and mediating inflammatory reactions [12], and the process of such damage can be clearly understood by observing blood metabolic indicators in patients. For example, low density lipoprotein cholesterol (LDL-C) and high density lipoprotein cholesterol (HDL-C) have been proven to be closely related to inflammatory injury in cells, blood vessels and tissues [13]; however, neither of them have been proven to be associated with neurological dysfunction in patients. Referring to previous studies, we found that inflammatory factors and blood metabolism were significantly abnormal in injuries such as cerebral infarction, cerebral ischemia-reperfusion [14]. Therefore, we inferred that inflammatory responses may also mediate the process when AD patients develop neurological disorders. In order to confirm our views and provide reliable theoretical guidance for future clinical treatment of AD, we analyzed the relationship of CD with inflammatory factors and metabolism in patients with AD.
Materials and methods
Patient data collection
This study included 80 consecutive AD patients (research group, or RG) treated in our hospital May 2019 to May 2020 and 71 non-AD patients (control group, or CG). This study has been approved by the Ethics Committee of our hospital, and the patients and their families have signed the informed consent.
Inclusion and exclusion criteria
Inclusion criteria: Patients were examined by the Tianjin Huanhu Hospital and found to meet the diagnostic criteria for AD [15], with an age range of 60-80 years old and complete case data. Exclusion criteria: Patients with CD caused by other serious diseases; Patients with impaired organ function; Patients with a previous major medical history; Patients with cardiovascular and cerebrovascular diseases; Patients with drug contraindications; Patients who did not cooperate with the treatment; or referred patients.
All patients in the CG had no major medical history, and routine examination showed good physical performance without autoimmune diseases.
Methods
In both groups, 5 mL of fasting venous blood was collected in the early morning, stored in a freezer at -30°C and centrifuged, and the resulting serum was obtained for subsequent detection. Enzyme Linked Immunosorbent Assay (ELISA) was employed to determine C-reactive protein (CRP; Shanghai Guduo Biotechnology Co., Ltd., Cat. No. GD-S0135-B), interleukin-6 (IL-6; Chuzhou shinuoda Biological Technology Co., Ltd., Cat. No. SND-H1925) and IL-1β (Wuhan Yipu Biotechnology Co., Ltd., Cat. No. CK-E10083), HITACHI automatic biochemical analyzer was used to detect metabolic indicators (low density lipoprotein cholesterol [LDL-C], high density lipoprotein cholesterol [HDL-C], total cholesterol [TC], triglyceride [TG], fasting plasma glucose [FPG], systolic blood pressure [SBP] and diastolic blood pressure [DBP]), and one-way immunodiffusion assay was utilized to measure carrier proteins ApoE and ApoAI. All the experiments were completed by laboratory technicians in our hospital.
Outcome measures
The mini-mental state examination (MMSE) [16], with a full score of 30 points, was used to evaluate the cognitive function of patients in the two groups, and the score was negatively associated with the cognitive function of patients. The changes of inflammatory factors and metabolic indicators were compared; the correlation of MMSE with inflammatory factors and metabolic indicators was analyzed by Pearson correlation analysis.
Statistical methods
Data was expressed as mean ± standard error (SE), and SPSS 22.0 software was utilized for statistical analysis. Comparisons between groups were made by independent sample t test, and those among multiple groups were performed by one-way ANOVA and LSD post-hoc test. Correlation analyses were carried out by Pearson correlation coefficient. Differences with p-values <0.05 were considered significant.
Results
Comparison of clinical data
The comparison of clinical data revealed no distinct difference in age, BMI, sex, course of disease, living environment, educational level and ethnicity between the two groups (P>0.05) Table 1.
Table 1.
Comparison of clinical data
| Research group (n=80) | Control group (n=71) | χ2 or t/P | |
|---|---|---|---|
| Age (years old) | 65.7 ± 5.4 | 66.2 ± 5.8 | 0.548/0.584 |
| BMI (KG/cm2) | 24.3 ± 1.3 | 24.5 ± 1.4 | 0.910/0.364 |
| Sex | 0.139/0.700 | ||
| Male | 52 (65.00%) | 44 (61.97%) | |
| Female | 28 (35.00%) | 27 (38.03%) | |
| Course of disease (year) | 4.35 ± 1.23 | 4.26 ± 1.25 | 0.445/0.657 |
| Living environment | 0.140/0.708 | ||
| Urban | 43 (53.75%) | 36 (50.70%) | |
| Rural | 37 (46.25%) | 35 (49.30%) | |
| Education level | 0.030/0.985 | ||
| Junior high school | 37 (46.25) | 32 (45.07) | |
| Senior high school or technical secondary school | 28 (35.00) | 25 (35.21) | |
| Junior college or above | 15 (18.75) | 14 (19.72) | |
| Ethnicity | 0.267/0.606 | ||
| Han | 73 (91.25%) | 63 (88.73%) | |
| Ethnic minorities | 7 (8.75%) | 8 (11.27%) |
Comparison of cognitive function scores
The cognitive function assessed by the mini-mental state examination (MMSE) scale identified evidently lower MMSE scores in the RG than in the CG (P<0.05) Figure 1.
Figure 1.
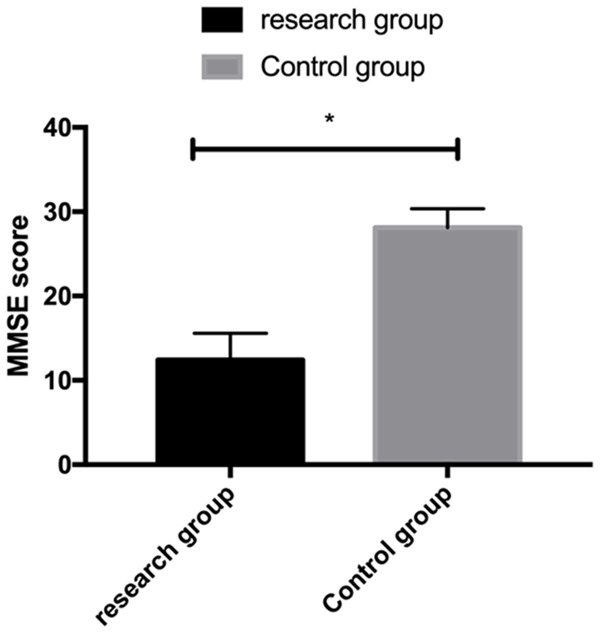
MMSE scores for the evaluation of cognitive function of patients in the two groups. *P<0.05.
Comparison of inflammatory factors
Measurement of inflammatory factors showed that IL-6, CRP and IL-1β were significantly higher in the RG than in the CG (P<0.05) Figure 2.
Figure 2.
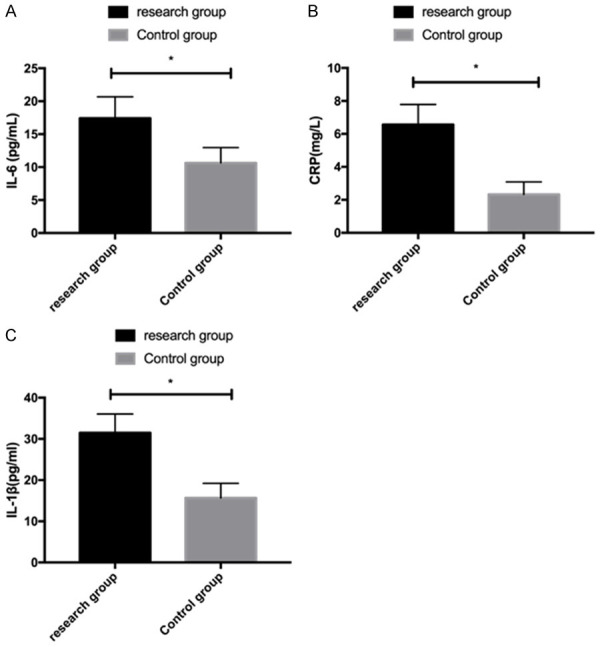
Inflammatory factors in the two groups. A: Comparison of IL-6 levels between the two groups. B: Comparison of CRP levels between the two groups. C: Comparison of IL-1β levels between the two groups. *P<0.05.
Comparison of metabolic indicators
When comparing the levels of metabolic indicators, it was found that LDL-C, TC, TG, FPG and SBP were higher while HDL-C was lower in the RG as compared to the CG (all P<0.05); however, no distinct difference was observed in DBP (P>0.05) Figure 3.
Figure 3.
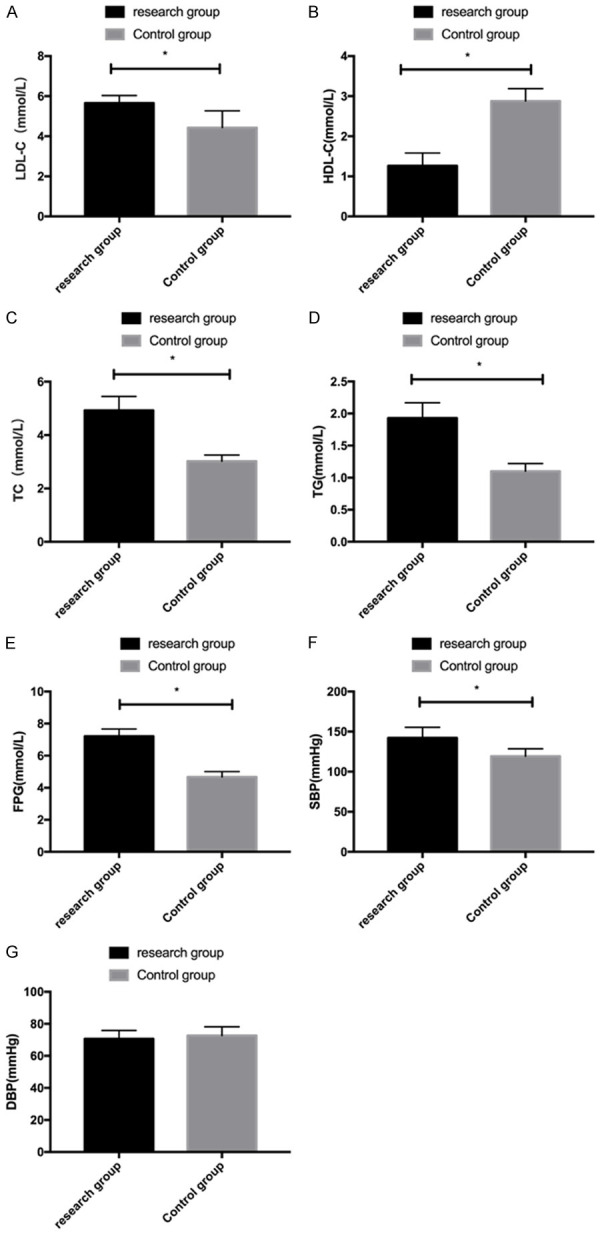
Comparison of metabolic indexes between the two groups. A: Comparison of LDL-C levels between the two groups. B: Comparison of HDL-C levels between the two groups. C: Comparison of TC levels between the two groups. D: Comparison of TG levels between the two groups. E: Comparison of FPG levels between the two groups. F: Comparison of SBP levels between the two groups. G: Comparison of DBP levels between the two groups. *P<0.05.
Comparison of carrier protein levels
Measurement of carrier protein levels exhibited that carrier proteins ApoE and ApoAI were lower in the RG than in the CG (P<0.05) Figure 4.
Figure 4.
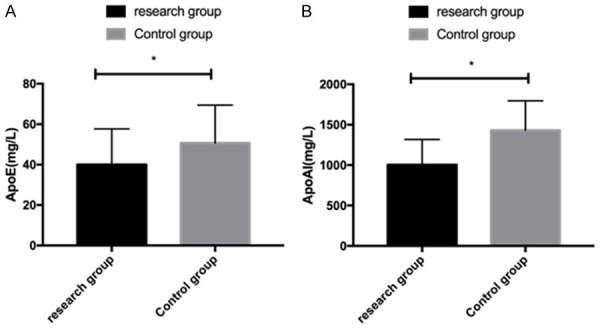
Comparison of carrier protein levels between the two groups. A: Comparison of ApoE levels between the two groups. B: Comparison of ApoAI levels of carrier proteins between the two groups. *P<0.05.
Correlation analysis between MMSE scores and inflammatory factors
Pearson correlation analysis showed that the MMSE score was negatively related to IL-6, CRP and IL-1β (r=-0.705, r=-0.723, r=-0.701, P<0.001) Figure 5.
Figure 5.
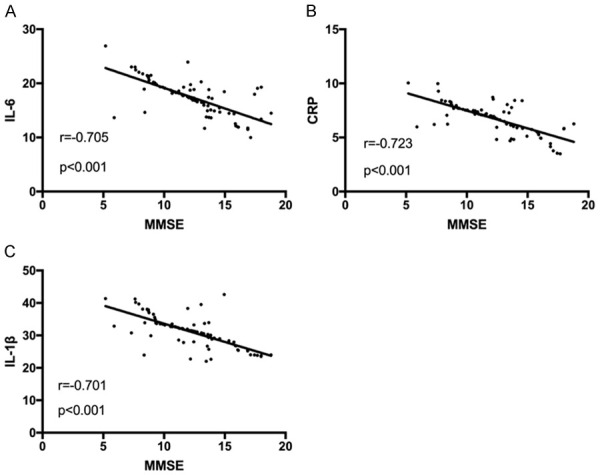
Correlation analysis between MMSE scores and inflammatory factors. A: Correlation between MMSE scores and IL-6 levels. B: Correlation between MMSE scores and CRP levels. C: Correlation between MMSE scores and IL-1 levels.
Correlation analysis between MMSE scores and metabolic indicators
Pearson correlation analysis indicated that the MMSE score was negatively associated with LDL-C, TC, TG, FPG and SBP (r=-0.595, r=-0.573, r=-0.696, r=-0.711, r=-0.678, P<0.001) Figure 6.
Figure 6.
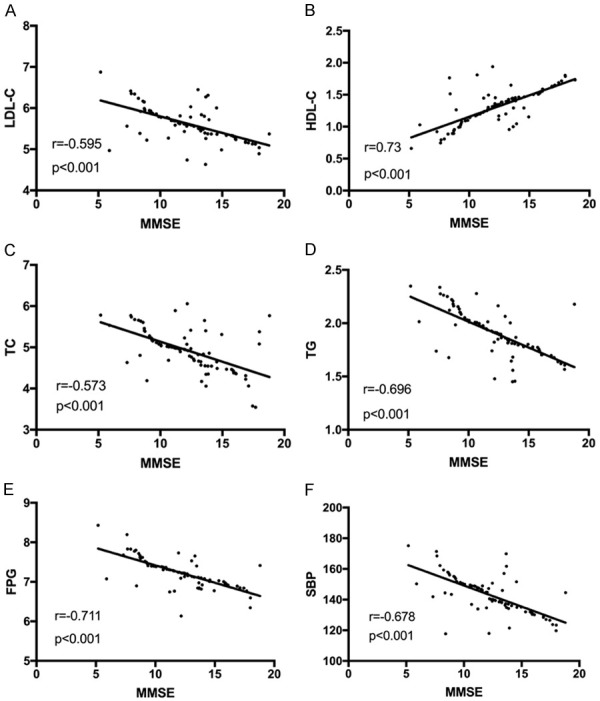
Correlation analysis between MMSE scores and metabolic indexes. A: Correlation between MMSE scores and LDL-C levels. B: Correlation between MMSE scores and HDL-C levels. C: Correlation between MMSE scores and TC levels. D: Correlation between MMSE scores and TG levels. E: Correlation between MMSE scores and FPG levels. F: Correlation between MMSE scores and SBP levels.
Correlation analysis between MMSE scores and carrier protein levels
Pearson correlation analysis showed that the MMSE score was negatively correlated with ApoE and ApoAI levels (r=0.584, r=0.662, P<0.001) Figure 7.
Figure 7.
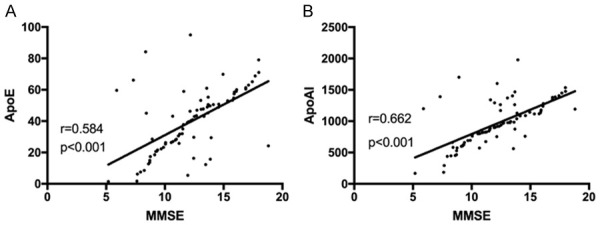
Correlation analysis between MMSE scores and carrier protein levels. A: Correlation between MMSE scores and ApoE levels. B: Correlation between MMSE scores and ApoAI levels.
Discussion
AD is a complex hereditary disease and it is the most common disease associated with age [17]. The global prevalence of AD is estimated to be as high as 24 million, which is expected to double every 20 years [18]. As the world’s population ages, the number of people at risk for AD is increasing [19]. AD is characterized by progressive cognitive decline, and it usually begins with impaired ability to form recent memories, which affects all intellectual functions [20] and leads to premature regression of basic functions that are completely required for daily life, seriously affecting the daily life of patients and their families [21]. Previous research suggests that besides environmental and genetic factors, abnormal levels of inflammatory factors and metabolic indicators are also closely related to the occurrence of AD [22]. Therefore, we analyzed the correlation of CD with inflammatory factors and metabolic indicators of AD patients in this study, with the hope of providing valuable reference for future clinical diagnosis and treatment of AD.
First, we compared the general data of patients such as age, BMI, sex, course of disease, living environment, educational level and ethnicity, and found no statistical difference, which confirmed that the two groups were appropriate to carry out comparative experiments. Then, we used the MMSE to analyze patients’ cognitive function, and found evidently lower scores in the RG than in the CG. The occurrence of AD is the result of the combined action of genes, lifestyle and environmental factors, and the resulting early primary clinical manifestation is CD [23]. In this paper, the MMSE was significantly decreased in the RG, suggesting that the MMSE score is closely related to the occurrence and progression of AD. Subsequently, we measured inflammatory factors IL-6, CRP and IL-1β, and determined notably higher levels in the RG than in the CG. CRP is a protein that rises sharply in the plasma during infection or tissue damage and reflects the level of inflammation in humans [24], while IL-1β and IL-6 are major cytokines involved in the inflammatory response [25]. In fact, inflammation will have adverse effects on the body, leading to disorders of the immune system. A large number of studies have reported the correlation between inflammation and diseases like cancer and cardiovascular diseases [26]. Other research has shown that inflammation also plays an important role in neurodegenerative diseases [27]. For instance, Author Luan YY found that the elevation of inflammatory factors contribute to neurodegenerative disease [28], which supports the results of this experiment and suggests that the changes of inflammatory factors have an important influence on CD in AD patients. Elevated TC and TG will cause damage to vascular endothelial tissue, lead to blocked blood flow and accelerated atherosclerosis, thus affecting brain metabolism [29]. Other evidence has pointed out that people with hyperlipidemia are at increased risk of developing AD [30]. LDL-C has been proven to be correlated with the incidence and severity of cardiovascular diseases, and is considered to be a main pathogenic factor for atherosclerosis. This study also found that in comparison with controls, LDL-C, TC, TG, FPG and SBP were higher in AD patients while HDL-C was lower. Among them, FPG is fasting plasma glucose, which is the most commonly used index of diabetes. It reflects the function of pancreatic islet B cells and generally represents the secretion function of basal insulin. Previous literature suggests that insulin changes may be closely related to the pathogenesis of AD [31], and another study pointed out that arterial systolic pressure is associated with the risk of AD [32], further suggesting that metabolic indicators exert a certain influence on early CD in AD patients. Carrier proteins are shown to affect most metabolic indicators in many neurodegenerative diseases and can be important factors in lipid metabolism [33]. In the present study, lower ApoE and ApoAI levels in AD patients were determined in AD patients, indicating that carrier proteins are closely related to AD and CD. To further confirm the correlation of CD in AD patients with inflammatory factors, metabolic indicators and carrier proteins, we applied Pearson correlation for analysis. The results exhibited that the MMSE score was negatively correlated with IL-6, CRP, IL-1β, LDL-C, TC, TG, FPG and SBP, while was positively associated with HDL-C, ApoE and ApoAI. It suggests that the decrease of MMSE score may lead to the increase of IL-6, CRP, IL-1β, LDL-C, TC, TG, FPG and SBP, and the decrease of HDL-C, ApoE and ApoAI. All in all, this investigation confirmed that CD in AD is closely related to the changes of inflammatory factors, metabolic indicators and carrier protein levels, which further proves that inflammatory and metabolic indicators as well as carrier proteins play an extremely important role in the development of AD. Moreover, the correlation between them may also be of vital importance in evaluating the disease changes of AD in the future.
AD is a chronic disease that is extremely difficult to cure completely and has a very long recovery cycle. However, due to the limited experimental conditions and short follow-up time, we were unable to evaluate the longterm prognosis of the patients in the RG. In addition, as a chronic disease with high incidence worldwide, CD and AD are not only related to inflammatory factors and metabolic abnormalities, so it cannot be ruled out that there are other factors that have a greater impact on AD patients or have more significant clinical application value. We will conduct more in-depth experimental exploration as soon as possible to address these limitations and obtain more complete experimental results for clinical reference.
Conclusion
To sum up, inflammatory factors and metabolic indicators are closely related to early CD in patients with AD, which may be excellent potential indicators for future diagnosis and treatment of AD.
Disclosure of conflict of interest
None.
References
- 1.Selkoe DJ. Alzheimer disease and aducanumab: adjusting our approach. Nat Rev Neurol. 2019;15:365–366. doi: 10.1038/s41582-019-0205-1. [DOI] [PubMed] [Google Scholar]
- 2.Butterfield DA, Halliwell B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat Rev Neurosci. 2019;20:148–160. doi: 10.1038/s41583-019-0132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morley JE, Farr SA, Nguyen AD. Alzheimer disease. Clin Geriatr Med. 2018;34:591–601. doi: 10.1016/j.cger.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 4.Jagust W. Imaging the evolution and pathophysiology of Alzheimer disease. Nat Rev Neurosci. 2018;19:687–700. doi: 10.1038/s41583-018-0067-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Congdon EE, Sigurdsson EM. Tau-targeting therapies for Alzheimer disease. Nat Rev Neurol. 2018;14:399–415. doi: 10.1038/s41582-018-0013-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henstridge CM, Hyman BT, Spires-Jones TL. Beyond the neuron-cellular interactions early in Alzheimer disease pathogenesis. Nat Rev Neurosci. 2019;20:94–108. doi: 10.1038/s41583-018-0113-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gotz J, Bodea LG, Goedert M. Rodent models for Alzheimer disease. Nat Rev Neurosci. 2018;19:583–598. doi: 10.1038/s41583-018-0054-8. [DOI] [PubMed] [Google Scholar]
- 8.Long JM, Holtzman DM. Alzheimer disease: an update on pathobiology and treatment strategies. Cell. 2019;179:312–339. doi: 10.1016/j.cell.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding Y, Sohn JH, Kawczynski MG, Trivedi H, Harnish R, Jenkins NW, Lituiev D, Copeland TP, Aboian MS, Mari Aparici C, Behr SC, Flavell RR, Huang SY, Zalocusky KA, Nardo L, Seo Y, Hawkins RA, Hernandez Pampaloni M, Hadley D, Franc BL. A deep learning model to predict a diagnosis of Alzheimer disease by using (18)F-FDG PET of the brain. Radiology. 2019;290:456–464. doi: 10.1148/radiol.2018180958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panza F, Lozupone M, Logroscino G, Imbimbo BP. A critical appraisal of amyloid-beta-targeting therapies for Alzheimer disease. Nat Rev Neurol. 2019;15:73–88. doi: 10.1038/s41582-018-0116-6. [DOI] [PubMed] [Google Scholar]
- 11.Munzel T, Sorensen M, Gori T, Schmidt FP, Rao X, Brook FR, Chen LC, Brook RD, Rajagopalan S. Environmental stressors and cardio-metabolic disease: part II-mechanistic insights. Eur Heart J. 2017;38:557–564. doi: 10.1093/eurheartj/ehw294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lopez-Candales A, Hernandez Burgos PM, Hernandez-Suarez DF, Harris D. Linking chronic inflammation with cardiovascular disease: from normal aging to the metabolic syndrome. J Nat Sci. 2017;3:e341. [PMC free article] [PubMed] [Google Scholar]
- 13.Balotsev R, Koido K, Vasar V, Janno S, Kriisa K, Mahlapuu R, Ljubajev U, Parksepp M, Veiksaar P, Volke V, Lang A, Haring L, Zilmer M, Vasar E. Inflammatory, cardio-metabolic and diabetic profiling of chronic schizophrenia. Eur Psychiatry. 2017;39:1–10. doi: 10.1016/j.eurpsy.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Ren Z, Zhao A, Wang Y, Meng L, Szeto IM, Li T, Gong H, Tian Z, Zhang Y, Wang P. Association between dietary inflammatory index, C-reactive protein and metabolic syndrome: a cross-sectional study. Nutrients. 2018;10:831. doi: 10.3390/nu10070831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cebi M, Babacan G, Oktem Tanor O, Gurvit H. Discrimination ability of the short test of mental status (STMS) compared to the mini mental state examination (MMSE) in the spectrum of normal cognition, mild cognitive impairment, and probable Alzheimer’s disease dementia: the turkish standardization study. J Clin Exp Neuropsychol. 2020;42:450–458. doi: 10.1080/13803395.2020.1758633. [DOI] [PubMed] [Google Scholar]
- 16.Rambeau A, Beauplet B, Laviec H, Licaj I, Leconte A, Chatel C, Le Bon P, Denhaerynck J, Clarisse B, Frenkiel N, Lange M, Joly F. Prospective comparison of the montreal cognitive assessment (MoCA) and the mini mental state examination (MMSE) in geriatric oncology. J Geriatr Oncol. 2019;10:235–240. doi: 10.1016/j.jgo.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 17.Ferretti MT, Iulita MF, Cavedo E, Chiesa PA, Schumacher Dimech A, Santuccione Chadha A, Baracchi F, Girouard H, Misoch S, Giacobini E, Depypere H, Hampel H Women’s Brain Project and the Alzheimer Precision Medicine Initiative. Sex differences in Alzheimer disease - the gateway to precision medicine. Nat Rev Neurol. 2018;14:457–469. doi: 10.1038/s41582-018-0032-9. [DOI] [PubMed] [Google Scholar]
- 18.Sweeney MD, Sagare AP, Zlokovic BV. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol. 2018;14:133–150. doi: 10.1038/nrneurol.2017.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi Y, Holtzman DM. Interplay between innate immunity and Alzheimer disease: APOE and TREM2 in the spotlight. Nat Rev Immunol. 2018;18:759–772. doi: 10.1038/s41577-018-0051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamazaki Y, Zhao N, Caulfield TR, Liu CC, Bu G. Apolipoprotein E and Alzheimer disease: pathobiology and targeting strategies. Nat Rev Neurol. 2019;15:501–518. doi: 10.1038/s41582-019-0228-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J, Gu BJ, Masters CL, Wang YJ. A systemic view of Alzheimer disease - insights from amyloid-beta metabolism beyond the brain. Nat Rev Neurol. 2017;13:612–623. doi: 10.1038/nrneurol.2017.111. [DOI] [PubMed] [Google Scholar]
- 22.Basu A, Du M, Sanchez K, Leyva MJ, Betts NM, Blevins S, Wu M, Aston CE, Lyons TJ. Green tea minimally affects biomarkers of inflammation in obese subjects with metabolic syndrome. Nutrition. 2011;27:206–213. doi: 10.1016/j.nut.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verheijen J, Sleegers K. Understanding Alzheimer disease at the interface between genetics and transcriptomics. Trends Genet. 2018;34:434–447. doi: 10.1016/j.tig.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 24.Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front Immunol. 2018;9:754. doi: 10.3389/fimmu.2018.00754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ng A, Tam WW, Zhang MW, Ho CS, Husain SF, McIntyre RS, Ho RC. IL-1beta, IL-6, TNF-alpha and CRP in elderly patients with depression or Alzheimer’s disease: systematic review and meta-analysis. Sci Rep. 2018;8:12050. doi: 10.1038/s41598-018-30487-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olsen RS, Nijm J, Andersson RE, Dimberg J, Wagsater D. Circulating inflammatory factors associated with worse long-term prognosis in colorectal cancer. World J Gastroenterol. 2017;23:6212–6219. doi: 10.3748/wjg.v23.i34.6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stephenson J, Nutma E, van der Valk P, Amor S. Inflammation in CNS neurodegenerative diseases. Immunology. 2018;154:204–219. doi: 10.1111/imm.12922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luan YY, Yao YM. The clinical significance and potential role of C-reactive protein in chronic inflammatory and neurodegenerative diseases. Front Immunol. 2018;9:1302. doi: 10.3389/fimmu.2018.01302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsoupras A, Lordan R, Zabetakis I. Inflammation, not cholesterol, is a cause of chronic disease. Nutrients. 2018;10:604. doi: 10.3390/nu10050604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao XS, Wu Q, Peng J, Pan LH, Ren Z, Liu HT, Jiang ZS, Wang GX, Tang ZH, Liu LS. Hyperlipidemia-induced apoptosis of hippocampal neurons in apoE(-/-) mice may be associated with increased PCSK9 expression. Mol Med Rep. 2017;15:712–718. doi: 10.3892/mmr.2016.6055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferreira LSS, Fernandes CS, Vieira MNN, De Felice FG. Insulin resistance in Alzheimer’s disease. Front Neurosci. 2018;12:830. doi: 10.3389/fnins.2018.00830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Heus RAA, Olde Rikkert MGM, Tully PJ, Lawlor BA, Claassen JAHR NILVAD Study Group. Blood pressure variability and progression of clinical Alzheimer disease. Hypertension. 2019;74:1172–1180. doi: 10.1161/HYPERTENSIONAHA.119.13664. [DOI] [PubMed] [Google Scholar]
- 33.Lieberman AP, Shakkottai VG, Albin RL. Polyglutamine repeats in neurodegenerative diseases. Annu Rev Pathol. 2019;14:1–27. doi: 10.1146/annurev-pathmechdis-012418-012857. [DOI] [PMC free article] [PubMed] [Google Scholar]


