FIGURE 1.
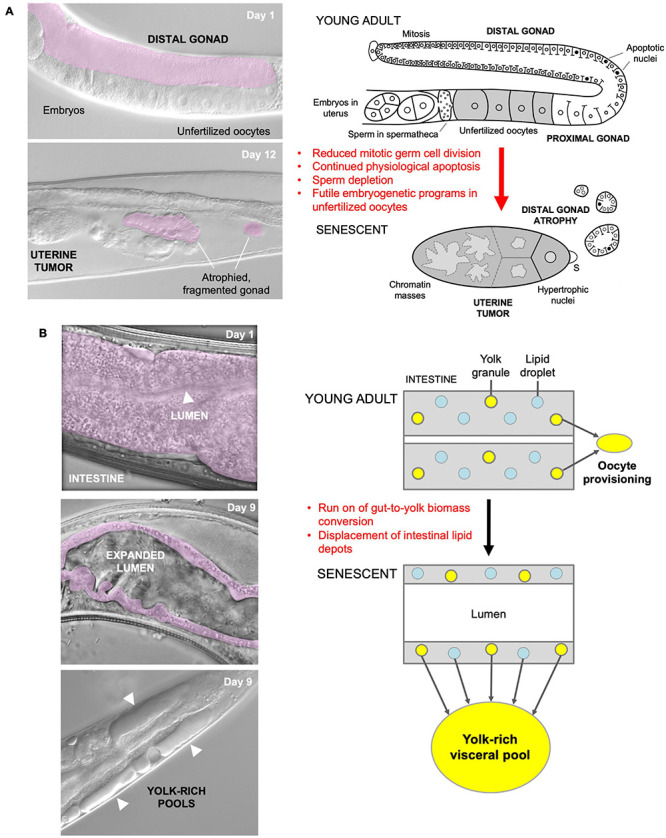
Quasi-programs as a cause of C. elegans hermaphrodite senescence. (A) Distal gonad atrophy and teratoma-like uterine tumor formation. Left: appearance of pathologies under Nomarski microscopy; distal gonad marked in pink. Right: proposed pathophysiology involving quasi-programs. In the young adult oocytes are generated by proliferation of mitotic germline stem cells which then enter meiosis, and then in most cases undergo physiological apoptosis (PA) to generate cytoplasm to fill expanding oocytes (Gumienny et al., 1999; Jaramillo-Lambert et al., 2007; Wolke et al., 2007). Subsequently, declining stem cell division (conceivably adaptive) (Kocsisova et al., 2019) and run-on of PA promotes distal gonad atrophy and fragmentation (de la Guardia et al., 2016). Unfertilized oocytes fail to complete meiosis, enter the uterus and develop into teratoma-like tumors containing massively polyploid chromatin masses (Golden et al., 2007) which appears to result, as in mammalian ovarian teratomas, from embryonic quasi-programs (Wang et al., 2018a,b). (B) Left, intestinal atrophy and yolk-rich visceral pool accumulation. Right, hypothesis for etiology of both pathologies: a vitellogenic quasi-program, where remobilization of intestinal biomass into yolk continues in a futile fashion (Ezcurra et al., 2018; Benedetto and Gems, 2019).
