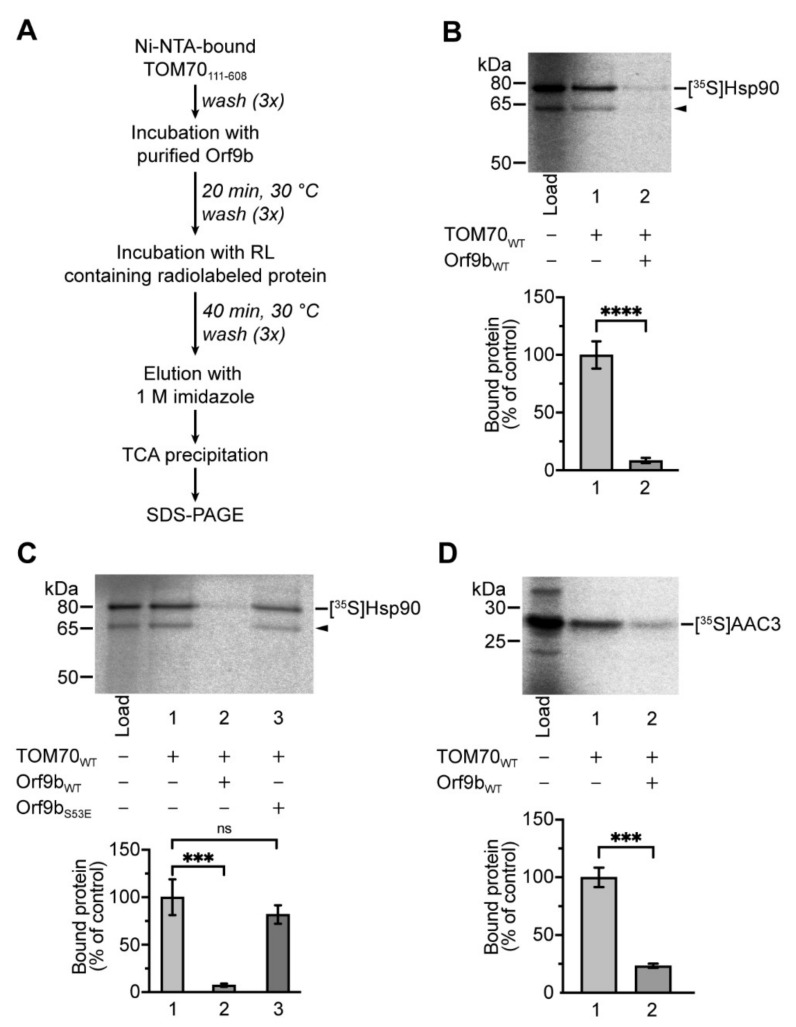
Figure 3.
Orf9b inhibits the binding of native Hsp90 to TOM70 in a phosphorylation-dependent manner. (A) Workflow of the binding assay used to investigate the effects of pre-incubation with Orf9b variants on the binding of native TOM70111–608 interactors. (B) Influence of Orf9b binding to TOM70 on the recognition of Hsp90. Ni-NTA-bound TOM70111–608 (50 pmol) was pretreated either with a 10-fold molar excess of isolated Orf9bWT or with buffer for 20 min at 30 °C and washed three times before addition of reticulocyte lysate (RL) containing in vitro-synthesized human [35S]Hsp90. Samples were incubated for 40 min at 30 °C and subjected to SDS-PAGE. The arrow indicates a second translation product of Hsp90. Binding of Hsp90 to TOM70111–608 without the addition of Orf9b was set to 100% (control). Signals obtained after SDS-PAGE and autoradiographic detection were quantified using 5% of the total added lysate as internal control (“Load”). **** indicate p-values < 0.0001, error bars show SEM, n = 6. (C) Binding of [35S]Hsp90 to Ni-NTA-bound TOM70111–608, pretreated with either buffer, isolated Orf9bWT, or isolated Orf9bS53E was performed as described in (B). Signals obtained after SDS-PAGE and autoradiographic detection were quantified using 5% of the total added lysate as internal control (“Load”). Binding of [35S]Hsp90 without Orf9b pre-incubation was set to 100% (control). *** indicate p-values < 0.001, “ns” indicates no significance, error bars show SEM, n = 4. (D) In vitro binding of [35S]AAC3 to Ni-NTA-bound TOM70111–608 pre-incubated with or without Orf9bWT. The experiment was carried out as described in (B). Signals obtained after SDS-PAGE and autoradiographic detection were quantified using 10% of the total added lysate as internal control (“Load”). Binding of [35S]AAC3 without Orf9bWT pre-incubation was set to 100% (control). *** indicate p-values < 0.001, error bars show SEM, n = 3.