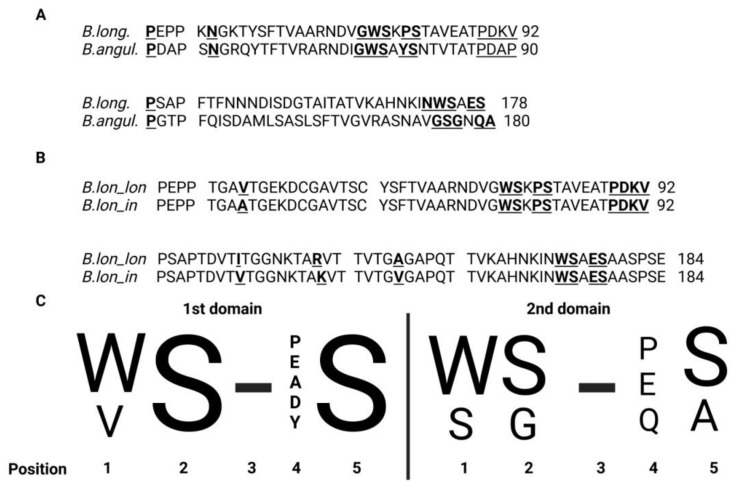
Figure 4.
(A) Alignment of sequences of protein fragments containing two FN3 domains derived from B. longum GT15 and B. angulatum GT102 (identity 40.1%; similarity 68.7%). Cytokine motifs in these species of bifidobacteria exhibited the starkest differences. The amino acids interjected between the domains are underscored. Amino acids in the “Cytokine receptor motif” site are highlighted by a double under-score. Amino acids in the “Interdomain contacts” site are highlighted in bold and underlined. (B) Alignment of sequences of protein fragments containing two FN3 domains from B. longum subsp. longum and B. longum subsp. infantis (identity 97.8%; similarity 100%). Differences in amino acid sequences in the first domain of FN3: 43 V → A; in the second domain FN3: 101 I → V, 109 R → K and 132 A → V. The amino acids interjected between the domains are underscored. Amino acids in the “Cytokine receptor motif” site are highlighted by a double under-score. Amino acids in the “Interdomain contacts” site are highlighted in bold and underlined. (C) This logo diagram resulted from aligning the corresponding aminoacid sequences of the gut-dwelling species B. longum, B. adolescentis, B. bifidum, B. breve, B. catenulatum, B. pseudocatenulatum, B. dentium, B. angulatum, B. kashiwanohense and B. gallicum. The motif in the second domain marked by an arrow is unique to B. angulatum. Any amino acid can be in position 3. Adapted from [60].