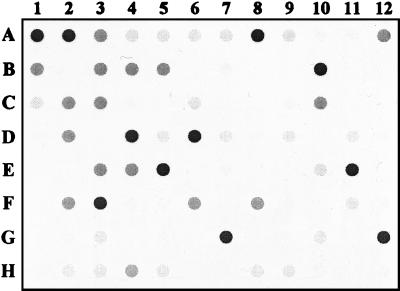
FIG. 1.
Random screening of schistosomiasis patients by FDA. The assay allows a semiquantitative reading; i.e., faint spots (1+ or 2+ color intensity) are considered weak positive and dark spots (3+ or 4+ color intensity) are considered strong positive. Wells A1 to C1 represent positive controls, and wells D1 to H1 represent negative controls.