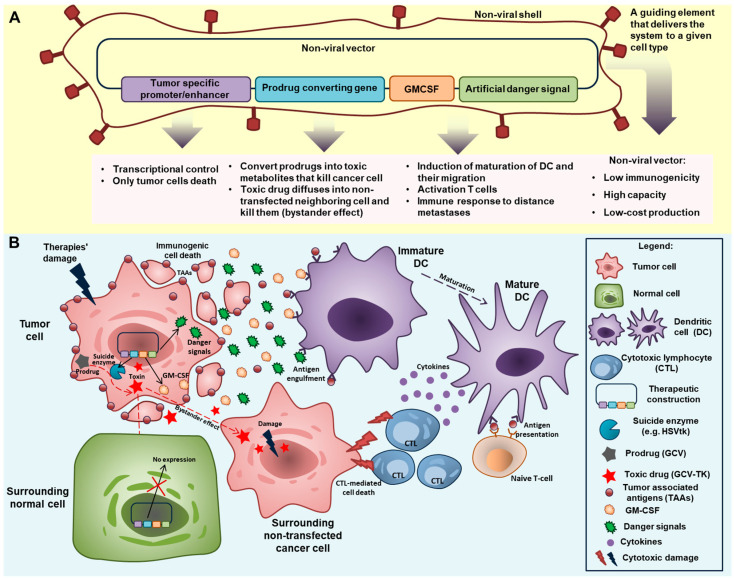
Figure 3.
The “ideal” GDEPT system. (A) Components for improving the GDEPT system vector. A plasmid vector that encodes the suicide enzyme converting the prodrug to a toxin under the control of the tumor-specific promoter alongside GM–CSF plus an artificial danger signal as immunomodulators. The plasmid vector is coated by a non-viral shell with a guiding element that targets the system for a given cell type. (B) Mechanism of action of the “ideal” GDEPT system. A therapeutic construction administered intratumorally transfects the tumor cells because the guiding element of the non-viral shell and tumor-specific promoter/enhancer transgenes are restricted to expression in normal cells. In transfected tumor cells, the prodrug-converting gene (suicide enzyme gene) starts to be expressed and convert the prodrug (GCV or 5-FC) into toxic compounds (GCV-TK or 5-FU). The toxins cause apoptosis of the tumor cells and diffuse to the surrounding non-transfected tumor cells. Furthermore, this type of suicide gene therapy and its combination with chemo-/radiotherapies causes immunogenic cell death, thereby eliciting an immune response. Tumor-associated antigens are then released through immunogenic cell death and can activate dendritic cells and induce an adaptive antitumor response. GM–CSF and the danger signal co-expressed with the prodrug-converting gene enhance the activation of immunity. This combined impact leads to the induction of DC maturation and the migration of DCs to the tumor, the activation of T-cells, and the development of an immune response to distant metastases. All these factors lead to the effective destruction of tumor cells.