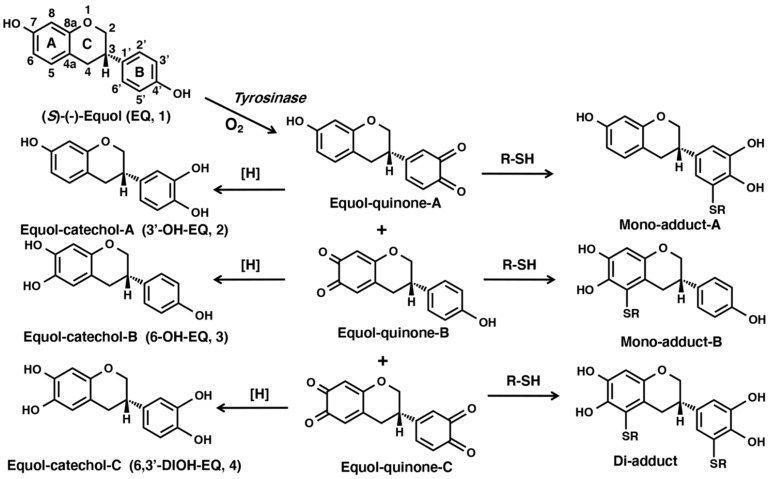
Figure 1.
Scheme showing the tyrosinase-catalyzed oxidation of (S)-(−)-equol (EQ, 1) in the absence or presence of a thiol. The oxidation of (S)-(−)-EQ 1 gives EQ-quinone-A and -B as immediate products, which are then oxidized to EQ-quinone-C. EQ-quinones are reduced by NaBH4 or L-ascorbic acid (AA) to form EQ-catechols, as shown by [H]. The tyrosinase-catalyzed oxidation of (S)-(−)-EQ 1 in the presence of a thiol, NAC, CySH, or GSH affords two mono-adducts and one di-adduct.