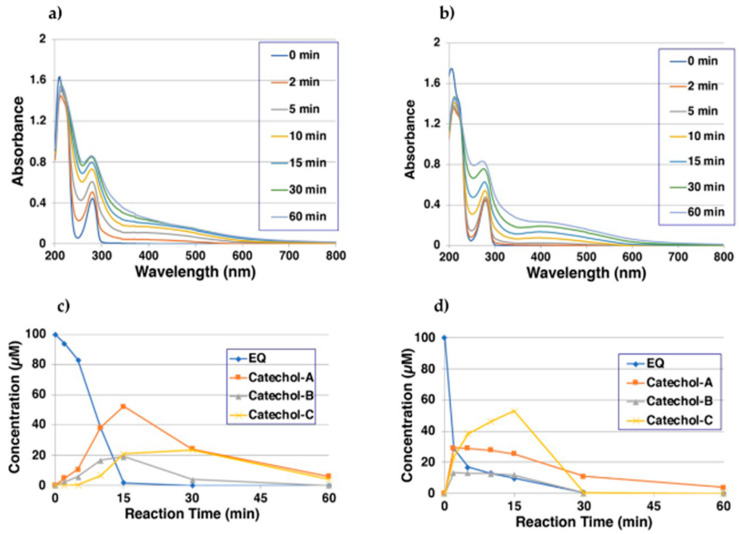
Figure 2.
Time course of the tyrosinase-catalyzed oxidation of EQ 1 and HPLC analyses of reaction products. (a) UV/visible spectral changes of EQ 1 at pH 6.8; (b) UV/visible spectral changes of EQ 1 at pH 5.3; (c) HPLC analysis following the tyrosinase-catalyzed oxidation of EQ 1 at pH 5.3, the reaction being stopped by the addition of NaBH4, followed by HClO4; (d) HPLC analysis following the tyrosinase-catalyzed oxidation of EQ 1 at pH 5.3 in the presence of 10 mol eq. AA, the reaction being stopped by the addition of HClO4. Data for (a,b) were obtained from single experiments, but reproducibility was confirmed for each experiment. Data for (c,d) were obtained from averages of two independent experiments.