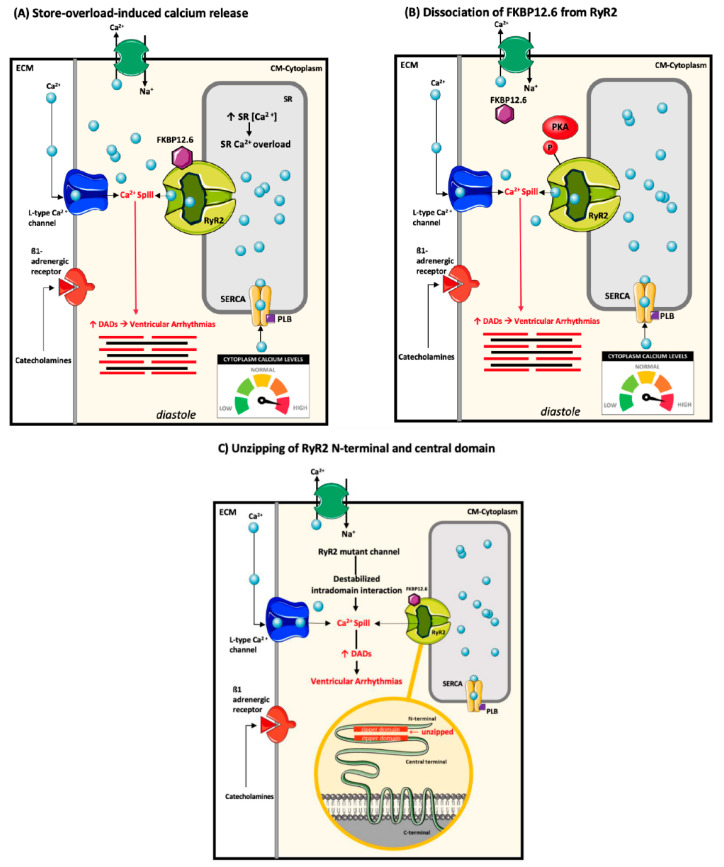
Figure 3.
Proposed mechanisms of RYR2-associated CPVT arrhythmogenesis: (A) Store overload-induced Ca2+ release: RyR2 mutant channels may exhibit an increased sensitivity to the luminal Ca2+ in the SR by decreasing the threshold needed to activate it, leading to increased Ca2+ release from the SR during diastole. This leads to delayed afterdepolarizations (DADs) and thus, uncoordinated activation of cardiac tissue and triggered ventricular arrhythmias. (B) FKBP12.6 dissociation from the RyR2-complex: FKBP12.6 is phosphorylated by protein kinase A, leading to its dissociation from mutant RyR2, increasing diastolic Ca2+ release and consequent contractility. (C) Unzipping of the N-terminal and central terminal of the RyR2 protein: N-terminal and the central domain of wildtype RyR2 interact to form a tight intramolecular structure or a “zip” to stabilize the channel. However, RyR2 mutant channels cause weak intramolecular domain interaction resulting in Ca2+ spill. P= phosphate group; Other abbreviations as indicated in Figure 2. Image adapted from Wleklinski, M. J., Kannankeril, P. J., and Knollmann, B. C. (2020). The Journal of Physiology, 598(14), 2817–2834 [60].