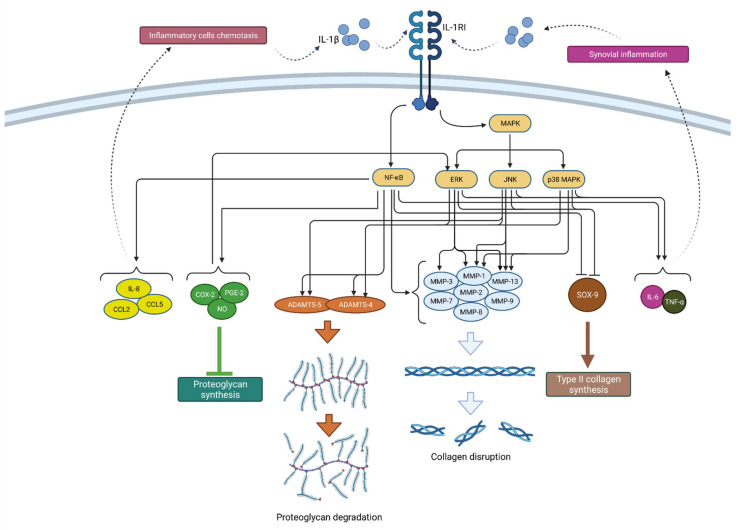
Figure 2.
Schematic representation of IL-1β function in osteoarthritis pathogenesis. By binding to its receptor (IL-1RI), IL-1β activates signaling pathways (NF-κB and MAPK) that, by raising the expression of enzymes (ADAMTS and MMPs), lead to catabolic reactions, i.e., proteoglycan degradation and collagen disruption. Furthermore, via the same signaling pathways, IL-1β inhibits type II collagen synthesis through SOX-9 suppression but also proteoglycan synthesis by increasing the synthesis of COX-2, PGE-2 and NO. In addition, IL-1β increases the expression of chemokines such as IL-8, CCL2 and CCL5, as well as the cytokines IL-6 and TNF-α, which attract inflammatory cells and cause synovial inflammation, respectively, resulting in the even greater production and secretion of IL-1β. IL-1β—interleukin 1β; IL-1RI—interleukin 1 receptor 1; MAPK—mitogen-activated protein kinase; ERK—extracellular signal-regulated kinases; JNK—c-Jun N-terminal kinases; NF-κB—nuclear factor kappa-light-chain-enhancer of activated B cells; MMPs—matrix metalloproteinases (MMPs); ADAMTS—a disintegrin-like and metalloproteinase with thrombospondin motif; COX-2—cyclooxygenase-2; PGE—prostaglandin E2; NO—nitric oxide; IL-8—interleukin 8; CCL2—chemokine ligand 2; CCL—chemokine ligand 5; SOX-9—SRY-Box Transcription Factor 9; IL-6—interleukin 6; TNF-α—tumor necrosis factor α.