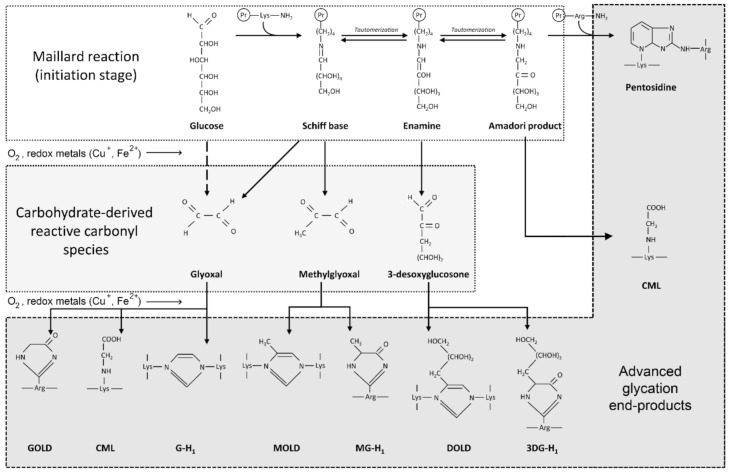
Figure 1.
Schematic representation of the mechanisms of AGE formation through the Maillard reaction and reactive carbonyl mediated protein modification. Briefly, the reaction between the protein free amino group and carbonyl group of a reducing carbohydrate (glucose) results in formation of a Schiff base. The latter is subsequently rearranged to a more stable Amadori product that yields AGEs including carboxymethyllysine (CML) or pentosidine in a series of reactions. In addition, carbohydrate-derived reactive carbonyls that are formed from glucose and Schiff bases also interact with protein molecules resulting in formation of numerous AGEs including glyoxal-derived di-lysine imidazolium crosslink (GOLD), CML, glyoxal-derived hydroimidazolone (G-H1), methylglyoxal-derived di-lysine imidazolium crosslink (MOLD), methylglyoxal-derived hydroimidazolone (MG-H1), desoxyglucosone lysine dimer (DOLD) and 3-deoxyglucosone-derived hydroimidazolone 1 (3DG-H1). Formation of AGEs as well as carbohydrate-derived carbonyl species is stimulated by redox metals including copper and iron, which are involved in Fenton chemistry, as well as by oxidative stress.