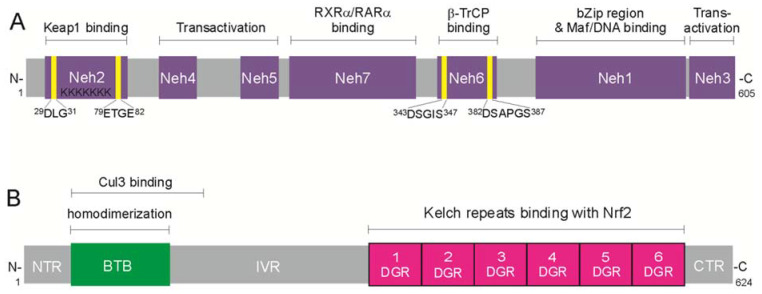
Figure 1.
Multi-domain organization of Nrf2 transcription factor and Kelch-like ECH-associated protein 1 (Keap1)—the repressor of Nrf2. (A) A 605-amino acid Nrf2 contains seven functional domains. The N-terminal Neh2 has two 29DLG31 and 79ETGE82 motifs that bind the Keap1 homodimer, which suppresses Nrf2 and mediates its ubiquitin-dependent proteasomal degradation; the Neh4 and Neh5 recruit transcriptional co-activators, CREB-binding protein (CBP), and/or repressor-associated coactivator (RAC); the Neh7 domain mediates repression of Nrf2 by retinoid X (RXR) and retinoic acid (RAR) receptors; the Neh6 has two 343DSGIS347 and 382DSAPGS387 motifs interacting with β-transducin repeat-containing protein (β-TrCP) and is responsible for the β-TrCP-mediated proteasomal degradation; the Neh1 contains a basic region-leucine zipper motif and is responsible for dimerization with small musculoaponeurotic fibrosarcoma (Maf), or BTB and CNC homology (Bach) proteins, the heterodimeric partners for Nrf2 to recognize the ARE sequence in target gene promoters; the C-terminal Neh3 domain is a transactivation domain that recruits chromodomain helicase DNA-binding domain protein 6 (CHD6). (B) Keap1 protein, the repressor of Nrf2, comprises five functional domains: the NTR domain in N-terminal region; the BTB domain, essential for homodimerization and for binding with Cul3-Rbx1 ligase complex; the intervening region (IVR), containing cysteine residues sensitive to oxidation; the double-glycine repeats (DGR)/Kelch domain, containing six Kelch-repeats, which operates as the binding sites for Nrf2; and the C-terminal region (CTR).