Abstract
Simple Summary
Lung cancer is a leading cause of cancer-related death worldwide and in most cases, detection is usually late and treatment resistance is frequent. For that reason, it is necessary to find biomarkers that could improve the diagnosis and disease management. Exosomes are a type of microvesicles secreted by tumor cells to the medium, with important functions in tumor development. Their analysis can be of utility in diagnosis, including early diagnosis, prognosis, treatment election or follow-up. However, isolation and analysis are cumbersome and can affect the subsequent data information. In this review, we will discuss the recent advances in the role of exosomes in lung cancer and their utility as liquid biopsy, with special attention to isolating methods.
Abstract
Lung cancer is a leading cause of cancer-related death worldwide and in most cases, diagnosis is reached when the tumor has already spread and prognosis is quite poor. For that reason, the research for new biomarkers that could improve early diagnosis and its management is essential. Exosomes are microvesicles actively secreted by cells, especially by tumor cells, hauling molecules that mimic molecules of the producing cells. There are multiple methods for exosome isolation and analysis, although not standardized, and cancer exosomes from biological fluids are especially difficult to study. Exosomes’ cargo proteins, RNA, and DNA participate in the communication between cells, favoring lung cancer development by delivering signals for growth, metastasis, epithelial mesenchymal transition, angiogenesis, immunosuppression and even drug resistance. Exosome analysis can be useful as a type of liquid biopsy in the diagnosis, prognosis and follow-up of lung cancer. In this review, we will discuss recent advances in the role of exosomes in lung cancer and their utility as liquid biopsy, with special attention to isolating methods.
Keywords: lung cancer, biomarker, exosomes, isolation liquid biopsy, microvesicles
1. Lung Cancer and Exosomes
Lung cancer is a leading cause of cancer-related death worldwide with smoking being the main risk factor for this disease [1]. This cancer can be divided into two types of histology, small-cell lung cancer (SCLC), that comprises 15% of the cases, and non-small cell lung cancer (NSCLC), about 85% of all cases, of which adenocarcinoma and squamous cell carcinoma are the most common subtypes [2]. About 75% of patients are diagnosed at locally advanced or even metastatic stage, when the prognosis is considered grim with a 5-year survival rate of only 15%. Thus, lung cancer treatment would greatly benefit from early detection, as a delayed diagnosis will increase mortality risk.
The understanding of the molecular bases of lung cancer with the discovery of driver mutations, such as in epidermal growth factor receptor (EGFR) gene in lung adenocarcinoma [3], and the identification of immune checkpoints that regulate the tumor immune response [4] have allowed the development of new therapies. As a result, in recent years important progresses were made in the treatment with EGFR inhibitors, anaplastic lymphoma kinase inhibitors, or immune checkpoints inhibitors [3,4,5]. However, as in other malignancies, lung cancer is composed of different cell populations with varied molecular alterations resulting in tumor and microenvironment heterogeneity [6]. In fact, the initial predominant targetable alterations can become less abundant during the course of the disease due to the selection of resistant sub-clones. The identification of these molecular characteristics during the evolution of the disease is of paramount importance in order to develop an efficient therapeutic strategy suitable to each clinical situation.
Tumor biopsy is the gold standard diagnostic procedure for histologic and molecular analysis. However, it is not always feasible due to difficult access to the lesions, and neither performing repeated biopsies during the course of the disease due to the procedure invasiveness. Additionally, biopsy may not reflect tumor heterogeneity. For that reason, it is necessary to find biomarkers with enough sensitivity and specificity for early diagnosis and for a close monitoring of the disease, helping in the choice of the best therapy for a personalized medicine. An alternative option to tissue biopsy is to perform the analysis in liquid biopsy, where repeated sampling can be easily performed [7]. Liquid biopsy in cancer consists of the analysis of three types of tumor-derived material in biological fluids: circulating tumor cells (CTCs), tumor-derived extracellular vesicles (EV), mainly exosomes, and circulating tumor DNA (ctDNA) [8]. Of them, ctDNA has attracted the most attention and several guidelines already include its analysis for the management of NSCLC [9,10]. Furthermore, some of these treatments mentioned before have already been allowed in patients with actionable mutations even when detected only in ctDNA [11].
Exosomes are spherical virus-size microvesicles with a density of 1.13–1.21 g/mL that participate in local and systemic intercellular communication transferring bioactive molecules between cells (reviewed in [12]). In the case of tumor-derived exosomes, they contribute to creating a favorable environment for tumor progression [13]. Consequently, tumor-derived exosomes play an important role in tumor development. Therefore, their analysis can help to gain a deeper knowledge of tumor biology and they can even be targets for drug therapy or delivery [14]. In addition, exosomes are very attractive due to their potential role as cancer biomarkers that could improve the management of cancer patients in general, and specifically, in lung cancer patients. In this review, we will summarize the role of exosomes in lung cancer development and their role as biomarkers of diagnosis, prognosis and even treatment election and follow-up.
2. Exosome Biogenesis and Structure
Cells release several types of microvesicles to the medium that differ in size, cellular origin and cargo: exosomes (50–200 nm), ectosomes (100–1000 nm) and apoptotic bodies (500–5000 nm) [15,16]. Exosome biogenesis initiates with the formation of the multivesicular bodies (MVB) containing many intraluminal vesicles formed by invagination of the endosomal membrane [15,16] (Figure 1). During this process, different materials from the parent cell, such as DNA, mRNAs, microRNAs (miRNAs), non-coding RNAs, lipids and proteins are selectively and actively incorporated into them [17]. Their release to the medium occurs through the fusion of multivesicular bodies with plasmatic membrane [16]. All cells can actively secrete exosomes, but it seems to be especially abundant in the case of tumor cells, with an estimation of 20,000 vesicles in 48 h by a single cancer cell [18]. Low oxygen tension and the resulting acidity due to increased glycolysis, typical conditions found in the tumoral microenvironment, favor the secretion of exosomes by cancer cells [19]. Increased secretion of tumor derived exosomes has been observed during the process of cancer development [20]. These exosomes can access circulation, where they have a short half-life and are cleared from blood in 6 h [21]. For example, Rabinowits et al. [22] found that plasmatic exosome levels were higher in lung adenocarcinoma patients compared to controls, probably due to alterations in cellular physiology.
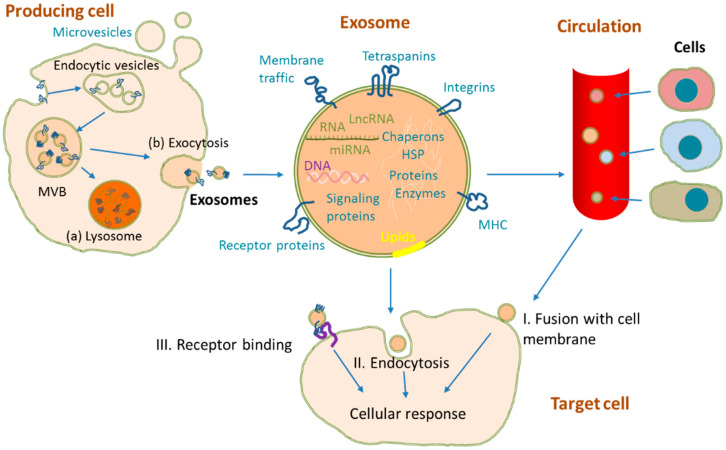
Figure 1.
Exosome biogenesis, release and uptake from other cells. Exosomes are formed by initial inward budding of plasma membrane and the formation on endocytic vesicles. Endosomal vesicles then form multivesicular bodies (MVB), which can either be degraded in lysosomes (a) or fused with the plasmatic membrane releasing exosomes to the medium (b). Other microvesicles can be formed and shed by simple outward budding of the cell membrane. During exosome formation, there is a selective incorporation of RNA, DNA, proteins and lipids, many of them characteristic of the producing cell. Released exosomes can reach circulation and interact with the target cell by fusion with either the cell membrane (I), endocytosis (II), receptor binding (III), or in combination, inducing intracellular signals.
Exosome membrane is a lipid bilayer especially enriched in lipid rafts, such as those of cholesterol, sphingomyelin and ceramide, which makes these microvesicles very stable and protected from degradative processes in the extracellular space [23]. Several thousand exosomal molecules have already been documented in different databases such as Exocarta database (http://www.exocarta.org, accessed on 7 July 2021). Surface proteins include tetraspanins (CD9, CD63 and CD81), integrins and adhesion molecules or ligands that can interact with specific receptors or cells. Other proteins included in exosomes are heat-shock proteins (HSP60, HSP70, etc.) as well as others involved in membrane transport and fusion (RAB5b, flotillin, annexins, etc.) or in MVB biogenesis (ALG2-interacting protein X, ALIX, and tumor susceptibility gene 101 protein, TSG101). Some of the proteins carried by exosomes are characteristic of the producing cells and can help to identify exosomal origin. For example, trophoblast exosomes can be identified by HLA-G transported in them [24], immune cells exosomes transport MHC I and II molecules [25] or T lymphocytes exosomes carry CD3 antigen [26]. Similarly, exosomal nucleic acid content is related to the type of producing cell in addition to the sorting process. Therefore, all this complex exosome composition helps to explain their many different roles in intercellular communication, and their potential utility as liquid biopsy.
3. Exosome Isolation and Identification
Exosomes have been obtained from different biological fluids, such as serum, plasma, urine, cerebrospinal fluid or exudates [27,28,29,30]. Exosome isolation methods are mainly based on their physicochemical properties, such as size or density, or their biological characteristics and molecules expressed in their surface [31,32]. These methods differ in efficiency, purity, and even in their capability to select exosome subpopulations [33,34]. A summary of isolation methods can be found in Table 1 including some of the commercial kits already available.
Table 1.
Methods for exosome isolation.
| Method | Isolation Principle | Assessment Parameters | Advantages | Disadvantages | Examples of Available Commercial Kits | References | ||
|---|---|---|---|---|---|---|---|---|
| Time | Purity | Recovery | ||||||
| Ultracentrifugation | Density by centrifugations at increasing speeds | +++ | + | + | Isolation of large volumes, no addition of chemicals, no pretreatment needed, most used method | Time consuming, expensive equipment, low purity, low reproducibility, damage of vesicles | [27,29,30,31,32,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49] | |
| Density gradient ultracentrifugation | Density by centrifugations in a density gradient | +++ | ++ | + | Effective in separation of EV from protein aggregates, high purity, no addition of chemicals | Time consuming, complex, low yields, fails to separate large vesicles with similar sedimentation rates | OptiPrep | [30,31,32,35,42,43,46,47] |
| Ultrafiltration | Size and molecular weight. Membranes with defined pore diameter or molecular weight cut offs | ++ | + | ++ | Simple and fast procedure, no special instrumentation, scalable | Clogging and trapping of vesicles on the filter, low yield, deformation of vesicles and lysis of exosomes, low purity | Amicon Ultra Centrifugal filters Vivaspin Centrifugal Concentrators |
[30,43,44,50,51,52,53,54,55] |
| Hydrostatic filtration dialysis | Size. Diffusion of particles across a porous membrane at concentration gradient | +++ | + | ++ | Simple, inexpensive, scalable, appropriate for diluted samples as urine | Selectivity of separation dependent on the cut-off, low purity | [29,56,57,58] | |
| Size exclusion chromatography | Size. Small particles penetrate a porous stationary phase and elute at different rates | ++ | ++ | ++ | Preserves vesicles integrity and biological activity, high recovery and reproducibility | Low yield, might require concentration, difficulty in scaling | Exo-spin qEV Extracellular Vesicle Isolation |
[27,33,34,39,43,44,47,52,53,59,60,61] |
| Asymmetric flow field-flow fractionation (AF4) | Size. Separation of particles in a channel with parabolic longitudinal flow combined with an external gradient | ++ | ++ | ++ | Possible EV subpopulation separation, possibility to couple to multidetection systems | Time consuming procedure, requires special equipment | [47,50,62,63] | |
| Immunoaffinity | Specific binding between antigens expressed on the exosome surface and corresponding antibodies | ++ | +++ | + | High purity and specificity, high selectivity, preservation of the activity of exosomal proteins, no protein contamination | Low yield, expensive, no scaling-up, EV cannot be readily eluted off the complexes with antibodies, antigenic epitopes might be blocked or masked | Dynabeads ExoFlow96 and 32 Exosome IP Kits ExoRNeasy Serum/Plasma Maxi Kit |
[31,32,33,39,45,46,47,64] |
| Precipitation with polymers | Change in either the solubility, aggregate formation or both, after reagent addition | ++ | + | +++ | High recovery, simple and fast procedure, no expensive equipment requirement, scalable | Low purity | ExoQuick Invitrogen Total Exosome Isolation Kit |
[33,34,35,39,43,45,47,48,49,65] |
| Microfluidics technology | Separation according to size, external markers or innovative sorting mechanisms such as acoustic, electrophoretic or electromagnetic fields | ++ | +++ | +++ | High purity and recovery, efficiency, minimal sample volume and reagent consumption, fast, reduce cross-contamination | Cost, additional equipment and complexity of devices | [39,66,67,68,69,70,71] | |
Assessment parameters: +: short/low; ++: medium; +++: long/high.
Within the methods based on physicochemical characteristics, ultracentrifugation procedures are the most widely and traditionally used to isolate exosomes, but isolated vesicles purity is low as other particles or protein aggregates can also sediment with them [72]. Besides, there are other important drawbacks such as its cost, the long and complex process, the difficulty to scale-up the number of samples, and to be used in clinical settings. Other methods are based on particle size, being the most common ultrafiltration and size exclusion chromatography [34,54]. Precipitation methods are based on the change of either their solubility, aggregate formation or both, after the addition of polyethylene glycol, protamine or sodium acetate [33,65]. Compared to ultracentrifugation procedures, these methods are less time consuming, do not require large volumes of biological samples or special equipment, and can be scalable. However, they lack specificity and isolated exosomes can also be accompanied by impurities. Another more specific method, but quite costly, is based on the interaction of antibodies with specific molecules at the exosome surface such as CD81, CD63 or CD9 [31,64]. When using immunocapture assays it should be considered that their efficiency depends on the number of exosomes, the density of antigen per particle and the antibody affinity for the exposed epitopes. In fact, not all antibodies that work properly with free molecules are suitable for exosome capture, as the target epitope might not be accessible due to the orientation or the folding of the protein in the exosome [73]. Another important issue is that exosomes can easily adhere to working material surfaces, with the consequent risk of either losing interesting exosomes, high background signal or both [13]. Finally, some protocols combine successive isolation and purification methods, such as polymer precipitation and immunoaffinity purification, rendering a fairly pure population of exosomes [33,74].
Plasma is one of the most complex fluids and isolated exosomes from it can be contaminated with particle aggregates, plasma proteins, such as albumin or fibrinogen, or with similar sized lipoproteins [33,75]. These contaminants can further affect functional and analytical studies. For example, lipoproteins may carry miRNA that could interfere when studying these molecules in exosomes [76]. Furthermore, proteomic analysis or even immunological analysis are prone to provide biased results due to these contaminants [33].
Although many data available are related to tumor exosomes from in vitro experiments, much less are related to tumor specific exosomes obtained from biological fluids. Blood usually contains high concentrations of exosomes, 108–1011 per mL, but most of them derive from blood cells, and tumor exosomes usually account for only a small proportion of the total circulating exosomes, making their isolation cumbersome [77]. The use of antibodies against exposed tumor antigens at the exosome membrane can be used to purify cancer exosomes [74]. This procedure can turn out well when specific tumor antigens exist, such as prostate specific antigen (PSA) in the case of prostate cancer exosomes, but this is not the case in lung cancer. Tumor marker MUC1, although not specific, is highly expressed in lung cancer exosomes [78], so it could be an antigen for their selective isolation by immunoaffinity. Alternatively, the epithelial cell surface molecule (EpCAM) is a frequent surface biomarker targeted for plasma tumor exosome enrichment in different epithelial cancers, including lung cancer [22,79].
Unfortunately, there is a lack of consensus on the best method, or even a standardized procedure for either exosome extraction, purification or both [80]. Related to this, the exosome isolation method should be taken into account when designing procedures as it might affect the subsequent experimental results [33]. For example, while in some experiments, it is necessary to recover the maximal amount of vesicles, and structure preservation and high purity are not necessary, in others on the contrary, the purity is of utmost importance. Other important points to consider are the starting volume and the possibility of scaling up the method, depending on the number of samples to be analyzed.
Depending on the isolation method, it is even difficult to unequivocally distinguish exosomes from other subgroups of microvesicles from the point of view of their size, density, morphology, or even biomarkers because these properties overlap between the different subclasses of microvesicles. Extracellular vesicles (EV) differentiation is difficult once released, and for this reason the International Society for Extracellular Vesicles (ISEV) recommends the use of the term extracellular vesicles when exosomes are not completely characterized [80].
Related to exosome characterization, the ISEV recommends using multiple complementary techniques to assess the results of extracellular vesicle-isolation methods (Figure 2) [80,81]. Exosomes can be identified by their size by transmission electron microscopy (TEM) or nanoparticle tracking analysis (NTA), which also allows measuring exosome concentration [82,83]. However, these methods do not distinguish exosomes from other nanoparticles with similar size. Specific exosomal proteins should be used as exosome biomarkers in combination with negative protein markers for better characterization. Membrane proteins, such as CD9, CD63 and CD81, or cytosolic proteins, such as TSG101, are frequent exosome markers detected by Western blot [80]. Purified EV should be quantified in terms of total particle number, protein or lipid content, in relation to the starting material. CD9, CD63 and CD81 have also been used for exosome quantification. Although they are expected to co-vary, CD63 can vary differently [84]. Furthermore, CD63 is under present in exosomes compared to cells, while CD81 can be up to 10-fold upregulated in exosomes [25].
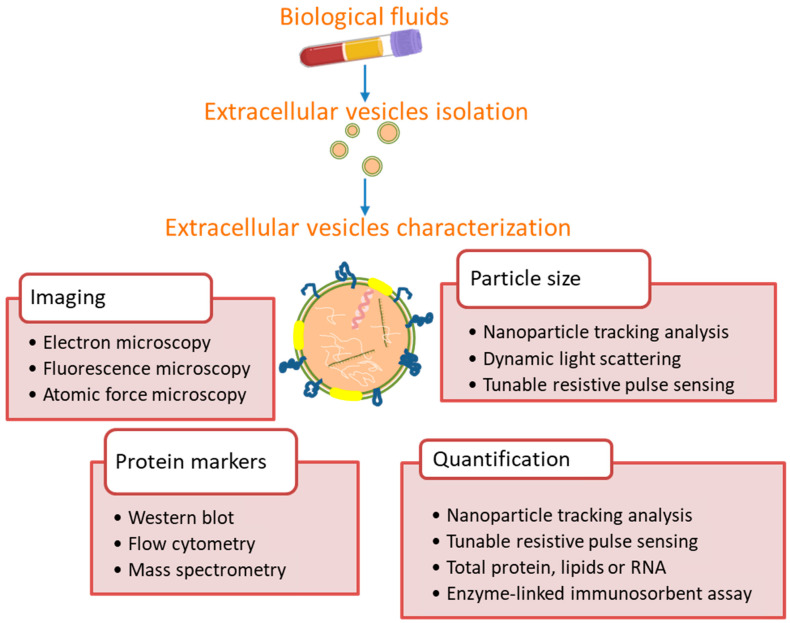
Figure 2.
Methods commonly used for extracellular vesicles characterization from biological fluids. According the ISEV [80,81], extracellular vesicles should be described by protein markers and single particle characterization by imaging or sizing, and also quantified in relation to the source material.
As mentioned before, it is important to define the source material and the isolation method, as they can have an important impact on results, and characterize the extracellular microvesicles in order to know the purity and recovery [80,85]. For example, Macias et al. [33] showed that biomarker detection varied depending on the purification method used and there was no correlation in the concentrations of exosomes obtained with different procedures. For these reasons, we indicate isolation and characterization methods in the following tables showing the clinical utility of exosome biomarkers.
4. Exosome Function in Lung Cancer
Secreted exosomes can be captured by other cells by fusion with plasma membrane, endocytosis, micropinocytosis, phagocytosis or receptor-mediated specific binding [86]. Carried material interacts with target molecules in recipient cells triggering a cellular response: exosome mRNA can be translated into proteins [87], miRNA and lncRNA can modulate gene transcription and mRNA translation in target cells [88,89,90], and exosomal proteins can interact with receptors [91,92]. These bioactive molecules can induce tumor growth and modify cancer microenvironment, thus favoring cancer progression and metastasis [93]. More concretely, exosomes have been implicated in crucial steps of cancer development, such as tumor proliferation, epithelial-mesenchymal transition (EMT), tumor migration and metastases, induction of angiogenesis and immunosuppression (Figure 3). In the following paragraphs, we will discuss some examples of these mechanisms in lung cancer.
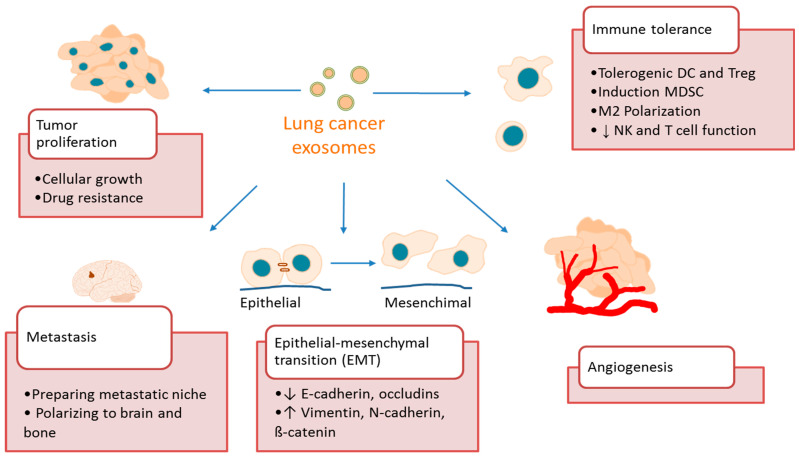
Figure 3.
Role of exosomes in lung cancer. Tumor exosomes participate in key steps of cancer progression, such as tumor cell proliferation, epithelial-mesenchymal transition, tumor migration and metastases, induction of angiogenesis and immune tolerance.
4.1. Exosomes Promote Lung Cancer Growth and Metastasis
Non-controlled cell proliferation is the basis of cancer growth and involves activation or altered expression of cell cycle genes and proteins. Tumor exosomes can carry molecules that can induce signals to stimulate tumor growth or even drive cell transformation [94]. miRNAs are the exosomal molecules that have probably been studied more extensively in relation to the different steps of the metastatic development. As an example, Wu et al. [95] showed that H1299 human lung adenocarcinoma cell line secretes miR-96-containing exosomes that inhibit the expression of LMO7, a tumor suppressor gene in lung cancer, and promote cell proliferation. Another study showed that A549 lung cancer adenocarcinoma cell line secretes exosomes engulfing miR-21 and miR-29a that bind Toll-like receptor TLR8 in immune cells, and trigger an NF-κB activation and secretion of inflammatory cytokines, thus favoring tumor growth and metastasis [88].
Mutations and gene amplifications of EGFR are important in NSCLC development and tyrosine kinase inhibitors (TKIs) have become a first-line therapy, although most patients relapse as drug resistance appears with time [2]. Different works revealed that exosomes could participate in the resistance to these drugs transferring miRNA or lncRNA from drug-resistant cancer cells to sensitive cells. For example, Zhang et al. [96] showed that gefitinib-resistant PC9 cells and their exosomes had high expression of miR-214. Those exosomes transferred miR-214 to sensitive PC9 cells that, as a result, acquired resistance. More recently, it was shown that exosomal transference of wild type EGFR promotes resistance to the TKI osimertinib by activating PI3K/AKT and MAPK signaling pathways [97]. Consequently, exosomes can also become therapeutic targets to overcome resistance development to these drugs.
An important step in tumor metastasis is EMT in which tumor cells lose their adherent characteristics of epithelial cells with decreasing expression of epithelial markers like E-cadherin and occludins, and acquire a mesenchymal phenotype with migratory and invasive capabilities, overexpressing mesenchymal markers like vimentin, N-cadherin o ß-catenin [98]. Different studies showed that exosomes participate in EMT in lung cancer transferring mesenchymal-induced signals and driving tumor cells to a more aggressive phenotype. For example, Rahman et al. showed that exosomes derived from highly metastatic lung cancer cells induced vimentin expression and EMT in HBE human bronchial epithelial cell line [99]. The highly metastatic lung cancer cell line SPC-A-1-BM and its exosomes were enriched in miR-499a-5p and by transferring this miRNA, these exosomes could increase the proliferation, migration and EMT via the mTOR pathway [100]. Cancer associated fibroblasts also secrete exosomes loaded with miR-210 that are uptaken by lung cancer cells inducing cell migration, proliferation, invasion abilities and EMT [89]. Finally, A549 cells, after TGF-β1-mediated EMT, release exosomes with cargo changes, both in protein and miRNA content, that induce further phenotypic changes via autocrine signaling [101].
An initial step for metastasis is the creation of a distant premetastatic niche with a favorable microenvironment where tumor cells can settle. Exosomes actively participate in this process, transporting active molecules in circulation that can modify target cells. In addition, through the carried molecules, particularly integrins, tumor exosomes can specifically target different organs or tissues and prepare the pre-metastatic niche [102]. Lewis lung carcinoma cell line produces exosomes containing miR-3473b, which once captured by lung fibroblasts cause NF-kB activation and inflammatory cytokines production, enhancing their intrapulmonary colonization [103]. Lung cancer commonly metastasizes to the brain and bone. Gang et al. [104] showed that lung cancer exosomes target brain microvascular endothelial cells inducing the release of Dkk-1 that provokes a displacement from M1 to a more pro-tumorigenic M2 phenotypic microglia. Subsequently, the metastatic lung cancer cells decrease Dkk-1 release removing the suppression on microglia that acquire a supportive phenotype. Furthermore, related to bone metastasis, Taverna et al. [91] observed that NSCLC exosomes contain amphiregulin, which binds EGFR in pre-osteoclasts activating the pathway that conducts to the expression of proteolytic enzymes initiating osteoclastic differentiation.
4.2. Exosomes Promote Lung Cancer Angiogenesis
Tumor growth is dependent on the blood supply with nutrients and oxygen, which requires the development of new vessels from the surrounding tissue. Tumor exosomes were shown to transport diverse molecules, especially miRNAs that once internalized by endothelial cells can induce neoangiogenesis. Hypoxia, which induces exosome release as we mentioned before, is very common in cancer and favors angiogenesis. For example, Hsu et al. [105] showed that lung cancer cells in hypoxic conditions secrete exosomes loaded with miR-23a, which once internalized in endothelial cells produces two effects in vasculature: first, it enhances angiogenesis by inhibiting prolyl hydroxylase 1 and 2, which produces accumulation of the hypoxia-inducible factor-1α (HIF-1 α); and secondly, it increases vascular permeability by inhibiting tight junction protein ZO-1 (zonula occludens 1 protein). Another study showed that exosomes from cigarette smoke extract-transformed human bronchial epithelial cells have high levels of miR-21 [94]. Exosomes transport this miR-21 into recipient normal human bronchial epithelial cells and induce elevated vascular endothelial growth factor (VEGF) levels promoting angiogenesis in human umbilical vein endothelial cells. Li et al. [106] observed that NSCLC cells overexpress leucine-rich-alpha2-glycoprotein 1 (LRG1), a protein that induces angiogenesis. Moreover, using the A549 cell line, they showed the release of exosomes loaded with LRG1, which induced, in endothelial cells, VEGF-A and angiopoietin-1 proangiogenic markers through a TGF-ß depended mechanism, and enhanced angiogenesis.
4.3. Exosomes Promote Lung Cancer Immune Tolerance
One of the central issues for tumor development is immune evasion through the development of a tolerogenic microenvironment avoiding cellular killing, thereby facilitating tumor progression. Tumor exosomes’ cargo can suppress immune cell function by two mechanisms in the target cell: either indirectly reprogramming of cells to suppress immune functions in other cells, or directly blocking immune function. Membrane associated HSP72 from tumor-derived exosomes can bind TLR2 ligand on myeloid-derived suppressor cells inducing a STAT3-dependent immunosuppressive function [92]. Huang et al. [107] showed that lung cancer exosomes induce dendritic cells into a tolerogenic phenotype and, secondarily, naïve CD4+ T cells into tumor antigen-specific regulatory T cells, which could suppress the tumor antigen specific CD8+ T cells. Similarly, another study showed that lung tumor cells under hypoxia secrete microvesicles packed with the TGF-ß and miR-23a, which in turn, inhibit NK cell function decreasing the cell surface expression of the activating receptor NKG2D and the cytotoxic marker CD107a/LAMP1, respectively [108]. A common mechanism of immune evasion is the upregulation of immune checkpoints molecules, such as programmed death-ligand 1 (PD-L1), which interact with their corresponding receptor in T cell, suppressing the response. Cheng et al. [109] showed that metastatic melanoma, breast and lung cancer cells release extracellular vesicles, mostly exosomes, expressing surface PD-L1, whose levels increased after IFN-γ stimulation. Microvesicle PD-L1 binds PD-1 in the surface of CD8 T cells and suppresses the function, thus favoring tumor growth.
5. Exosomes as Biomarkers in Lung Cancer
Given the exosomal content, potential biomarkers comprise a wide variety of molecules including proteins and nucleic acids (Table 2, Table 3 and Table 4) Although initially many of the studies focused on proteins, in the last few years, miRNAs have attracted growing attention [110]. In many cases, utility does not rely on a single molecule but on a panel of them instead. Related to this and as it occurs with many other aspects of biological research, bioinformatics and big-data analysis have become key players, allowing management and evaluation of huge quantities of data in the search for the best markers.
Table 2.
Studies on clinical utility of exosomal proteins as biomarkers in lung cancer.
| Molecule | Sample | Number of Subjects | Isolation Methods | Characterization Methods | Utility | Comments | Authors |
|---|---|---|---|---|---|---|---|
| CD151, CD171, and tetraspanin 8 | Plasma | 336 LC + 126 C | EV array | - | Diagnosis | AUC calculated between LC and controls and when subdividing in AC, SCC and SCLC. NYESO1, HER2, EGFRvIII, SFTPD, Florilin1, CD142 and Mucin 16 also analyzed | Sandfeld-Paulsen et al. [111] |
| CD91 (+CEA) | Serum | Screening set: 10 C, 10 IP, 14 AC, 12 SCC Validation set: 54 C, 19 IP, 105 AC, 34 SCC |
Immune-affinity for screening set ELISA with anti-CD9 in validation set |
- | Diagnosis | Screening set: isolation by immune-affinity with anti-CD9 tips and proteomic study to identify CD9 Validation Set: ELISA with anti CD9 as capture antibody and anti-CD91 as detection antibody |
Ueda et al. [112] |
| AHSG and ECM1 | Serum | 125 NSCLC + 46 C | Ultracentrifugation | TEM/NTA/WB | Diagnosis (including early stage) | Differentially expressed proteins identified by mass spectrometry | Niu et al. [113] |
| Panel of 30 proteins | Plasma | 109 advanced NSCLC + 110 C | EV Array | - | Diagnosis | Array for 37 proteins | Jakobsen et al. [84] |
| SRGN, TPM3, THBS1 and HUWE1 | Plasma | 13 AC + 15 C | Density gradient | TEM/NTA/WB | Diagnosis | 108 differentially expressed proteins identified by mass spectrometry | Vykoukal et al. [114] |
| CD5L, CLEC3B, ITIH4, SERFINF1, SAA4, SERFINC1, and C20ORF3 | Serum | 20 AC + 20 SCC + 20 SCLC + 20 C | Polyethylene glycol -based precipitation and immunoaffinity separation using antibodies against CD9, CD63, CD81, and EpCAM | TEM/NTA/DLS/WB | Diagnosis | Differentially expressed proteins identified by mass spectrometry; 55 confirmed by Western blot. CD5L highest AUC | Choi et al. [74] |
| LRG1 | Urine | 8 NSCLC + 10 C | Ultracentrifugation | TEM | Diagnosis | Differentially expressed proteins identified by mass spectrometry | Li et al. [115] |
| CD171 (1), NY-ESO-1 (2) | EDTA Plasma | 276 NSCLC | EV array | - | Prognosis: (1) OS, (2) HR | Array for 49 proteins | Sandfeld-Paulsen et al. [116] |
| PD-L1 | Plasma | 33 NSCLC | Precipitation | TEM/NTA/WB | Prognosis: OS and PFS | Quantification with Simoa Bead Technology | Yang et al. [117] |
| HSP70 | EDTA Plasma | 20NSCLC+ 14 C + 10 BC | Ultracentrifugation | NTA/TEM | Diagnosis, prognosis (metastasis detection), monitoring | HSP70 barely detected in plasma. Exosomal HSP70 correlates with tissue analysis | Chanteloup et al. [118] |
Abbreviations: AC: adenocarcinoma; AUC: area under ROC curve; BC: breast cancer; C: controls; CEA: carcinoembryonic antigen; CYFRA21-1: cytokeratin 19 fragment; DLS: dynamic light scattering; EDTA: ethylenediaminetetraacetic acid; EV: extracellular vesicles; HR: hazard ratio; IP: interstitial pneumonia; LC: lung cancer; NSCLC: non-small cell lung cancer; NTA: nanoparticle tracking analysis; OS: overall survival; P: pneumonia; PFS: progression-free survival; SCC: squamous carcinoma; SCLC: small cell lung cancer; T: tuberculosis; TEM: transmission electron microscopy; TNM: tumor-node-metastasis; WB: Western blot.
Table 3.
Studies on clinical utility of exosomal miRNAs as biomarkers in lung cancer.
| Molecule | Sample | Number of Subjects | Isolation Methods | Characterization Methods | Utility | Comments | Authors |
|---|---|---|---|---|---|---|---|
| (1) miR-378a, miR-379, miR-139-5p, and miR-200b-5p (2) miR-151a-5p, miR-30a-3p, miR-200b-5p, miR-629, miR-100, and miR-154-3p |
Plasma | Screening set: 10 AC+ 10 LG + 10 C Validation set: 50 AC+ 30 LG + 25 C |
Precipitation | - | (1) Diagnosis AC+ LG vs. C (2) Diagnosis AC vs. LG |
Wide-range miRNAs analysis (742 microRNAs) | Cazzoli et al. [119] |
| miR-9-3p, miR-205-5p, miR-210-5p and miR-1269a | Serum | Training set: 74 NSCLC + 74 C Validation set: 73 NSCLC + 75 C |
Precipitation | TEM/NTA/WB | Diagnosis | 10 miRNAs to be analyzed were selected previously from TCGA database | Wang et al. [120] |
| miR-5684 (1) and miR-125b-5p (1, 2, 3) +CEA |
Serum | 330 NSCLC + 312 C | Ultracentrifugation | TEM/tunable resistive pulse sensing/WB | (1) Diagnosis, (2) Prognosis: Metastasis detection and survival, (3) therapy monitoring | 22 miRNAs profiled by microarrays and verified by quantitative PCR | Zhang et al. [121] |
| miR-23b-3p + CEA + CYFRA21-1 | Serum | 80 NSCLC + 60 P + 30 C | Precipitation | TEM/NTA | Diagnosis Prognosis: tumor size, depth of invasion, liver metastasis and TNM stage |
Quantification by RT-PCR. miRNA-39 was used as the external reference gene | Wang et al. [122] |
| let-7f-5p (1) miR-320a, miR-622 and let-7f-5p (2) + CEA and CYFRA21-1 |
Plasma | 80 NSCLC + 30 C | Membrane affinity spin columns | - | (1) Diagnosis (2) Metastasis detection |
miRNA array | Wang et al. [123] |
| miR-20b-5p and miR-3187-5p | Serum | 276 NSCLC (104 stage I) + 282 C | Ultracentrifugation | TEM/NTA/WB | Diagnosis (including early stage) | miRNAs profiled by microarrays and verified by quantitative PCR | Zhang et al. [124] |
| miR-21/Let-7a ratio | Serum | 75 NSCLC + 23 BPN + 18 PID +24 C | Precipitation | - | Diagnosis (including versus benign and inflammatory lung diseases) | Quantification by RT-PCR | Yang et al. [125] |
| let-7, miR-21, miR-24, and miR-486 (1) miR-181-5p, miR-30a-3p, miR-30e-3p, and miR-361-5p (2) miR-10b-5p, miR-15b-5p, and miR-320b (3) |
Plasma | Testing set: stage I (16 AC + 10 SCC) + 12 C Validation set: stage I (10 AC + 10 SCC) + 30 C Symptomatic set 60 |
Ultracentrifugation + immune-affinity with anti-EpCAM beads | NTA/WB | (1) Diagnosis at early stage (2) Histological classification: AC (3) Histological classification: SCC |
Small RNA profile with RNA NGS and subsequent confirmation with RT-PCR. Normalization with cel-miR-39 | Jin et al. [126] |
| miR-4257 and miR-21 | EDTA Plasma | Screening set: 6 NSCLC Validation set: 129 stage I + 34 stage II +32 stage III + 30 C |
Ultracentrifugation | TEM | Histological classification Prognosis: TNM stage, tumor size, lymphatic invasion, disease-free survival |
miRNA selected with an array in 6 NSCLC patients (3 with and 3 without recurrence) | Dejima et al. [127] |
| miR-205-5p and miR-200b | Pleural effusion | 9 LC + 9 P + 9 T | Ultracentrifugation | TEM/NTA/WB | Diagnosis | Small RNA sequencing and subsequent confirmation with RT-PCR in 8 randomly chosen miRNAs | Lin et al. [128] |
| miR-429, miR-205, miR-200b, miR-203, miR-125b and miR-34b | Serum | Discovery set: 38 NSCLC + 16 COPD + 16 C Technical validation set: 16 NSCLC + 8 COPD + 6 C External validation set: 100 NSCLC + 58 C |
Precipitation | - | Diagnosis (including early stage) | 754 microRNAs screened with TaqMan Low Density Arrays. In the 10 miRNAs upregulated a technical validation was performed by RT-PCR. Global normalization was performed | Halvorsen et al. [129] |
| miR-182 and miR-210 | Pleural effusion | 41 AC + 15 BPE | Precipitation | - | Diagnosis | miR-21, miR-31, miR-182, and miR-210 analyzed by RT-PCR. Normalization with miR-16 | Tamiya et al. [130] |
| miRNA-205 | Urine and saliva | 5 LC+ 5 C | Fe3O4@SiO2-aptamer nanoparticles | WB | Diagnosis | Development of a POCT device | Zhou et al. [131] |
| miR-574-5p and miR-328-3p and miR-423-3p | Plasma | 30 NSCLC (16 with and 14 without bone metastasis) + 14 C | Ultracentrifugation | WB | Bone metastasis detection | Small RNA sequencing | Yang et al. [132] |
| miR-146a-5p | Serum | 100 NSCLC with cisplatin-based chemotherapy | Precipitation | TEM/NTA/WB | Chemotherapy resistance Prognosis |
Absolute miRNA levels quantify with RT-PCR with standard curves. Relative levels related to exosomal protein content | Yuwen et al. [133] |
| miR-1246 (1) and miR-96 (1,2,3) | Heparin Plasma | 52 NSCLC (27 Radioresistant + 25 radiosensitive) + 45 C | Lipid nanoprobe | TEM/NTA/WB | (1) Diagnosis (2) Radioresistance detection (3) Prognosis: OS |
miR-21, miR-1246, let-7g, miR-210, miR-214, and miR-96 analyzed by RT-PCR. Normalization with cel-miR-39 | Zheng et al. [134] |
| hsa-miR-320d, hsa-miR-320c, and hsa-miR-320b | Plasma | 5 NSCLC with partial response to PD-1/PD-L1 inhibitors + 4 with progression + 7 C | Ultracentrifugation | TEM | Response to PD-1/PD-L1 inhibitors | Small RNA profile with RNA NGS; 155 miRNAs differentially expressed versus controls | Peng et al. [135] |
Abbreviations: AC: adenocarcinoma; BPE: benign pleural effusion; BPN: benign pulmonary nodules; C: controls; CEA: carcinoembryonic antigen; COPD: chronic obstructive pulmonary disease; CYFRA21-1: cytokeratin 19 fragment; EV: extracellular vesicles; LC: lung cancer; LG: lung granuloma; miRNA: microRNA; NGS: next generation sequencing; NSCLC: non-small cell lung cancer; NTA: nanoparticle tracking analysis; OS: overall survival; P: pneumonia; PFS: progression-free survival; PID: pulmonary inflammation diseases; RT-PCR: real-time polymerase chain reaction; SCC: squamous carcinoma; SCLC: small cell lung cancer; T: tuberculosis; TCGA: The Cancer Genome Atlas; TEM: transmission electron microscopy; TNM: tumor-node-metastasis; WB: Western blot.
Table 4.
Studies on clinical utility of exosomal mRNAs, lncRNAs and circRNAs as biomarkers in lung cancer.
| Molecule | Sample | Number of Subjects | Isolation Methods | Characterization Methods | Utility | Comments | Authors |
|---|---|---|---|---|---|---|---|
| TP63, KRT5, CEACAM6 and SFTPB mRNAs | Serum | 54 AC + 16 SCC | Ultracentrifugation | TEM/NTA/WB | Histological classification | 17 miRNAs to be analyzed were selected previously from TCGA database as differentially expressed between AC and SCC. ACTB and SLC25A6 were used as internal references | Cao et al. [136] |
| eIF4E RNA | Serum | 99 NSCLC + 40 C | Precipitation | TEM/NTA/WB | Diagnosis Prognosis: stage, distant metastases, OS and PFS |
eIF4E data extracted from TCGA database | Dong et al. [137] |
| PD-L1 (1) and IFN-γ (1,2) mRNA | EDTA Plasma | 38 NSCLC | Membrane affinity spin columns | - | (1) Response to treatment (2) PFS |
Quantification by ddPCR with ACTB as internal control | Del Re et al. [138] |
| MALAT-1 | Serum | 77 NSCLC + 30 C | Precipitation | TEM/NTA/WB | Diagnosis Prognosis (Lymph node metastasis, TNM stage) |
Quantification by RT-PCR. GAPDH was used for normalization | Zhang et al. [90] |
| linc01125 | Serum | 277 NSCLC + 187 C + 5 P + 59 T + 58 COPD | Precipitation | - | Diagnosis Prognosis (stage, OS) |
RNA-Seq for lncRNA profile and subsequent quantification of linc01125 by RT-PCR with spiked in controls | Xian et al. [139] |
| FECR | Serum | 35 with limited SCLC and 26 with extensive SCLC +55 C | Affinity Chromatography | TEM/WB | Diagnosis Prognosis (survival) Response to chemotherapy |
RT-PCR with β-actin as control | Li et al. [140] |
| circ_0014235 and circ_0025580 | Plasma | 30 SCC + 30 C | Precipitation | - | Diagnosis Prognosis (TNM stage and tumor size) |
circRNA sequencing and confirmation with RT-PCR with GAPDH as internal control | Wang et al. [141] |
| circRNA_0056616 | EDTA plasma | 90 AC (42 with lymph node metastasis and 48 without) | Precipitation | TEM/WB | Lymph node metastasis predictor | RT-PCR. Normalization as Wang’s methods | He et al. [142] |
| circSATB2 | Serum | 83 NSCLC + 95 C | Ultracentrifugation | TEM/NTA/WB | Diagnosis Prognosis (metastasis detection) |
RT-PCR. GAPDH and U6 were used as internal references and cel-miR-39 as an external reference | Zhang et al. [143] |
| circ_0047921, and circ_0007761 (1) circ_0056285 (1,2) |
Serum | Screening set: 30 NSCLC + 45 C Training set: 120 NSCLC + 165 C Validation set 1: 62 NSCLC + 95 C Validation set 2: 63 NSCL + 58 COPD + 46 T |
Precipitation | TEM/NTA/WB/FC | (1) Diagnosis (including early stage) (2) Prognosis: state of progression and lymph-node metastases |
1701 circRNAs initially identified by RNA-seq, 17 of them were differentially expressed and 8 of them were validated by RT-PCR with GAPDH and ACTB as spiked-in controls | Xian et al. [144] |
Abbreviations: AC: adenocarcinoma; C: controls; circRNA: circular RNA; COPD: chronic obstructive pulmonary disease; ddPCR: droplet digital PCR; EV: extracellular vesicles; FC: flow cytometry; LC: lung cancer; miRNA: microRNA; NSCLC: non-small cell lung cancer; NTA: nanoparticle tracking analysis; OS: overall survival; P: pneumonia; PFS: progression-free survival; RT-PCR: real-time polymerase chain reaction; SCC: squamous carcinoma; SCLC: small cell lung cancer; T: tuberculosis; TCGA: The Cancer Genome Atlas; TEM: transmission electron microscopy; TNM: tumor-node-metastasis; WB: Western blot.
5.1. Exosomal Proteins
Some of the studies in exosomal protein profiles have been performed using arrays that allow a multiplex analysis of exosomal proteins but without requiring a previous exosomes isolation [145]. For example, Sandfeld-Paulsen et al. [111] found that CD151, CD171 and tetraspanin 8 presented significant differences between controls and multiple lung cancer histological types, although the associated areas under ROC curve (AUC) for individual markers were quite limited with a maximum AUC of 0.68. Only when combining 10 markers in a panel could the AUC reach 0.76. Interestingly, some of these proteins, such as CD91, presented better diagnostic efficiency in other studies when combined with carcinoembryonic antigen (CEA) [112]. In fact, this and other studies combine exosomal markers with classical serological markers to achieve better diagnostic efficiencies. For instance, in another proteomic study of exosomal content, Niu et al. [113] detected higher levels of alpha-2-HS-glycoprotein (AHSG) and extracellular matrix protein 1 (ECM1) in serum samples from NSCLC patients when compared with healthy volunteers. In the case of AHSG, all cancer patients taken into account, the associated AUC was 0.736, which was reduced when selecting only early-stage patients. Regarding ECM1, both AUCs were similar and lower than that of AHSG. However, when AHSG was combined with CEA, the AUCs increased to 0.938 and 0.911, respectively, in total and early-stage NSCLC patients, substantially improving the efficiency of CEA alone. Meanwhile, Jakobsen et al. [84] performed this type of analysis with 37 antibodies. When combining CD81, CD63 and TAG72, the multivariate analysis showed an AUC of 0.758. Only when up to 30 proteins were included in the analysis, did the AUC reach a value of 0.830. A larger study performed with mass spectrometry identified 108 proteins with differential expression in exosomes between lung adenocarcinoma patients and healthy controls. Four of them, SRGN, TPM3, THBS1 and HUWE1, presented a combined AUC of 0.90 [114]. Another recent study identified CD5L as another potential biomarker [74]. This protein, an apoptosis inhibitor, was found to be overexpressed in both exosomes and cancer tissues and presented an AUC for lung cancer diagnosis of 0.943.
The term liquid biopsy usually refers to peripheral blood samples but other fluids’ analyses are also possible. Of particular interest is urine, which has the advantage of being less complex than plasma, although there are other issues to consider, such as the contamination from urine proteins or the time of collection. In fact, a recent article was published with methodological considerations from the Urine Task Force of the International Society for Extracellular Vesicles for exosome analysis in urine samples [146]. A proteomic study of lung cancer patients revealed high expression of LRG1 in urinary exosomes and lung tissue, suggesting that this protein can be a potential biomarker [115]. However, the specificity for lung cancer is expected to be low as other cancers also express LRG1 [147,148].
Regarding prognosis, Sandfeld-Paulsen et al. [116] observed an association between NY-ESO-1, EGFR, PLAP, EpCam and Alix from plasma exosomes with poor overall survival, although only NY-ESO-1 kept the association when Bonferroni correction was applied.
EGFR gene evaluation has already become a key test in lung cancer management for prognosis and TKIs therapy election [149]. Additionally, EGFR protein evaluation in exosomes could also be of interest. For example, EGFR is present in 80% of exosomes purified from the lung cancer biopsies whereas in only 2% of exosomes from patients with chronic lung inflammation [107].
Another critical aspect of cancer management is therapy monitoring, and some exosomal biomarkers were also evaluated for this scope. For example, Yang et al. [117] showed that an increase in exosomal PD-L1 indicated response to treatment and better overall survival. Similarly, exosomal HSP70 is present in membranes of cancer-derived exosomes but not in exosomes from non-cancerous cells and correlate with HSP70 content within the tumor biopsies [118]. Moreover, HSP70 has been found useful not only in diagnosis and prognosis, but also in patients’ follow-up, with increasing levels in patients with disease progression and decreasing levels in those with partial or total response.
Table 2 shows a summary of recent clinical studies of the utility of exosomal proteins as biomarkers in lung cancer.
5.2. Exosomal miRNAs
As mentioned before, exosomal miRNAs have been implicated in lung cancer progression through multiple mechanisms including promotion of angiogenesis, vascular permeability and metastasis (reviewed in [150]). Some of these miRNAs were evaluated as biomarkers, mainly in diagnosis and prognosis (Table 3) [8]. In most cases, clinical utility does not rely on a specific miRNA but on a panel of multiple miRNAs. A combination of miR-151a-5p, miR-30a-3p, miR-200b-5p, miR-629, miR-100 and miR-154-3p achieved 96% sensitivity and 60% specificity in discriminating lung cancer from granuloma patients [119]. Wang et al. established a panel with four miRNAs (miR-9-3p, miR-205-5p, miR-210-5p, miR-1269a) that could discriminate NSCLC patients from healthy controls with an AUC of 0.91 (77% sensitivity and 89% specificity) [120]. Among them, miR-1269a presented the highest discriminatory capacity. Other candidates for being diagnostic biomarkers in early-stage NSCLC are miR-20b-5p and miR-3187-5p [124].
As exosomal proteins, miRNAs have also been combined with classical biomarkers of lung cancer. For example, miR-125b-5p’s usefulness has been evaluated as a diagnostic marker with discrete results when considering all stages (AUC = 0.700) and even lower when focusing on early-stage patients [121]. Its combination with CEA slightly improved CEA efficiency from 0.79 to 0.83. In addition, it could discriminate early versus advanced disease as well as the presence of lymph node and distant metastases. Another miRNA evaluated is miR-23b-3p that presented a diagnostic efficiency in ROC analysis of 0.915, much higher than those observed for classical serological markers such as CEA and CYFRA 21-1 [122]. Similarly, another study on miRNAs showed the utility in NSCLC diagnosis of let-7f-5p miRNA alone and in combination with CEA and CYFRA 21-1 [123]. Although diagnostic performance for NSCLC of the miRNA let-7f-5p is better compared to other conventional markers, it has a near perfect classification when combined with them, with an AUC of 0.981 (sensitivity of 94.7% and specificity of 93.3%).
In some cases, the utility does not rely on individual miRNAs’ levels but on the ratio between them instead, as in the miR-21/Let-7a ratio that clearly identified lung cancer patients from healthy volunteers (with sensitivity and specificity of 56% and 100%, respectively) and from those with pulmonary benign nodules (sensitivity and specificity were 56% and 82.6%, respectively) [125].
Exosomal miRNA analysis can help not only in diagnosis but also in histological classification. For example, Jin et al. [126] identified early-stage NSCLC patients with high sensitivity (80%) and greater specificity (92%) with a panel of miRNAs (let-7b-5p, let-7e-5p, miR-23a-3p and miR-486-5p). Moreover, these miRNAs allowed histological classification with miR-181b-5p and miR-361b-5p mainly being expressed in exosomes from adenocarcinoma patients, and miR-10b-5p and miR-320b in squamous cell carcinoma patients.
Multiple studies have analyzed both diagnostic and prognostic utility of exosomal miRNAs. For instance, Dejima et al. [127] evaluated miR-21 and miR-4257 and found, not only higher levels of both miRNAs in NSCLC patients, but also an association with clinical parameters such as tumor size and TNM stage in the case of miR-21, and with histological type, lymphatic invasion and TNM stage for miR-4257. Moreover, higher levels of both miR-21 and miR-4257 were associated with shorter disease-free survival.
Exosomal profile in pleural effusions can also be useful to detect lung malignancies. In fact, Lin et al. [128] observed that from 254 miRNAs detected in exosomes from pleural effusions, miR-205-5p and miR-200b could differentiate malignant effusions from those of pneumonia and tuberculosis patients. These two miRNAs were also included in a combination of exosomal miRNAs from peripheral blood (miR-429, miR-205, miR-200b, miR-203, miR-125b and miR-34b) that identified early-stage patients with a sensitivity of 85% and specificity of 74% [129]. More recently, Tamiya et al. [130] identified another pair of miRNAs, miR-182 and miR-210, which were able to identify malignant pleural effusions from benign ones with an AUC in ROC curves of 0.87 and 0.81, respectively.
Development of new and easy-to-use technological devices already allows the transfer of exosome research to clinical application. For example, a point-of-care device was recently developed to analyze salivary and urinary miR-205 [131], one of the miRNAs identified with diagnostic utility in some of the studies mentioned before.
About 20–40% of lung cancer patients develop bone metastasis with a negative impact in overall survival [151]. Yang et al. [132] identified three exosomal miRNAs with differential expression in NSCLC patients depending on whether they had bone metastasis or not. In the case of miR-574-5p, it was downregulated, while miR-328-3p and miR-423-3p were upregulated. All of them participate in the Wnt/β-catenin signaling pathway and thus can regulate metastasis development.
Exosomal miRNAs were also evaluated in the context of treatment election and follow-up. Regarding chemotherapy resistance, Yuwen et al. [133] observed that NSCLC patients with low expression of miR-146a-5p presented shorter progression-free survival. Moreover, the overexpression of miR-146a-5p could revert chemoresistance to cisplatin in A549 lung cancer cells by inhibiting autophagy. Regarding radiotherapy, Zheng et al. [134] showed that miR-96 can identify lung cancer patients with high efficiency (AUC = 0.97) but it also presents potential utility to identify radioresistant patients (AUC = 0.75). Another treatment option that has become a key strategy against lung cancer is immunotherapy, with PD-1/PD-L1 as one of the targeted checkpoints. Related to this, exosomes can also inform of the potential response to anti-PD-1 treatments. In a study from Peng et al. [135], a signature of three miRNAs from the miRNA-320 family could predict the efficacy of this type of therapy whereas downregulation of miR-125b-5p during treatment identifies patients in partial response.
5.3. Other Nucleic Acids
Although miRNAs are the most studied nucleic acids in exosomes, other molecules have also proved their utility as biomarkers in lung cancer patients (Table 4). In this way, Cao et al. [136] identified four mRNAs contained in exosomes that distinguished squamous carcinoma and adenocarcinoma. The combination of these mRNAs, tumor protein P63 (TP63), keratin 5 (KRT5), CEA cell adhesion molecule 6 (CEACAM6) and surfactant protein B (SFTPB), improved their histological classification capacity. Related to prognosis, exosomal eIF4E RNA was associated with TNM stage and the presence of metastases [137]. Furthermore, patients with higher levels presented shorter survival.
RNA analysis allows the detection of EML4-ALK fusion that identifies patients that would develop resistance to EGFR inhibitors and would be susceptible to being treated with ALK inhibitors. Brinkmann et al. has observed that exosomal RNA can reflect the fusion transcript observed in tissue, becoming a potential alternative when tissue biopsies are not an option [152]. Moreover, exosomal mRNAs could also serve as biomarkers of immunotherapy efficacy. For instance, Del Re et al. [138] showed that patients receiving nivolumab or pembrolizumab with high baseline IFN-γ mRNA levels in exosomes had shorter progression-free survival than those with lower levels. Similarly, patients that progressed within three months, presented higher levels than those that responded or had disease stabilization.
Exosomal long non-coding RNAs (lncRNAs) were also evaluated as potential clinical biomarkers for lung cancer management. For example, in 2017, Zhang et al. [90], showed the utility of MALAT-1 as a diagnostic biomarker of NSCLC. Moreover, this lncRNA presented prognostic utility as it correlated with tumor stage and lymphatic metastases. Since then, multiple lncRNAs’ utility was proved not only in diagnosis and prognosis, but also as therapy targets (reviewed in [153]). More recently, linc01125 could distinguish NSCLC cases from disease-free and tuberculosis controls and correlated with an unfavorable overall survival [139]. Related to these lncRNAs, there is even a meta-analysis evaluating their diagnostic capacity [154].
Given the recent finding of circular RNA, there are far fewer studies assessing their role in lung cancer, but some of them have proved their utility as potential biomarkers. For instance, Li et al. showed that FLI1 exonic circular RNA (FECR) was increased in SCLC patients and it was correlated with the metastatic status [140]. In another study, circ_0014235 and circ_0025580 presented diagnostic utility to identify squamous cell carcinoma patients, and were strongly correlated with higher TNM stage and tumor size [141]. circRNA_0056616 also proved its capability to identify patients with lymph node metastasis [142]. Similarly, circSATB2 could also detect lung cancer metastasis [143]. Xian et al. showed the diagnostic utility of a panel comprising three circRNAs, circ_0047921, circ_0007761 and circ_0056285, to differentiate NSCLC patients from not only healthy controls but also from patients with other types of pulmonary diseases. The latter, also presented prognostic utility [144].
6. Conclusions
Exosomes are important players in lung cancer development participating in tumor aggressiveness such as in metastasis, with organ polarization to brain and bone, angiogenesis, immune escape and even drug resistance. Due to their size and capability to transfer molecules into target recipient cells, exosomes also postulate as potential drug delivery vehicles [14]. As the tumor exosomal cargo includes molecules from the releasing cells and can be detected in circulation, exosomes can serve as non-invasive biomarkers providing a potential alternative or at least, a complementary tool to conventional biopsy with additional advantages in the diagnosis, prognosis, therapy election and follow-up. Furthermore, the analysis of the complex composition of exosomes can provide a multianalyte approach that could give a dynamic insight into the tumor microenvironment, helping to provide a more precise and rapid medical intervention. This is important, as the implementation of different therapeutic strategies, using new cancer drugs discovered in the last years, needs appropriate biomarkers for guidance. In addition, exosome analysis could help in the screening and early detection of lung cancer, when patients have better prognosis [113,124], and some clinical trials are already addressing this issue (www.clinicaltrials.gov). However, exosome analysis has not been included in clinical guides yet, contrary to ctDNA where there are already clinical indications for its use as biomarker in lung cancer and some commercial kits are already available for mutations’ assessment [8,155]. One of the reasons is the lack of standardized protocols for exosome isolation and analysis, which impairs the implementation of their analysis in a routine clinical laboratory. Sophisticated technology, but also affordable and easy-to-use, for exosome analysis would also help to implement their use. In addition, studies are usually retrospective and with small cohorts and thus, more prospective studies with larger populations are needed. Finally, it is difficult to select the exosome biomarkers that correlated better with the clinical situation between different reported studies. Once these issues are solved, exosomes will probably be key participants in lung cancer management. To achieve this, it is necessary to develop more translational research and clinical trials before introducing exosomes in the management of lung cancer.
Acknowledgments
The authors would like to thank María Romero for her support in the preparation of the manuscript.
Author Contributions
Writing—reviewing and editing, A.S., E.A. and Á.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2021. CA A Cancer J. Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 2.Herbst R.S., Morgensztern D., Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553:446–454. doi: 10.1038/nature25183. [DOI] [PubMed] [Google Scholar]
- 3.Tsao M., Sakurada A., Cutz J.-C., Zhu C., Kamel-Reid S., Squire J., Lorimer I., Zhang T., Liu N., Daneshmand M., et al. Erlotinib in lung cancer—Molecular and clinical predictors of outcome. N. Engl. J. Med. 2005;353:133–144. doi: 10.1056/NEJMoa050736. [DOI] [PubMed] [Google Scholar]
- 4.Gettinger S., Horn L., Jackman D., Spigel D., Antonia S., Hellmann M., Powderly J., Heist R., Sequist L.V., Smith D.C., et al. Five-year follow-up of nivolumab in previously treated advanced non–small-cell lung cancer: Results from the CA209-003 study. J. Clin. Oncol. 2018;36:1675–1684. doi: 10.1200/JCO.2017.77.0412. [DOI] [PubMed] [Google Scholar]
- 5.Leighl N.B., Rekhtman N., Biermann W.A., Huang J., Mino-Kenudson M., Ramalingam S.S., West H., Whitlock S., Somerfield M.R. Molecular testing for selection of patients with lung cancer for epidermal growth factor receptor and anaplastic lymphoma kinase tyrosine kinase inhibitors: American Society of Clinical Oncology Endorsement of the College of American Pathologists/International Association for the Study of Lung Cancer/Association for Molecular Pathology Guideline. J. Clin. Oncol. 2014;32:3673–3679. doi: 10.1200/jco.2014.57.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Izumchenko E., Chang X., Brait M., Fertig E., Kagohara L.T., Bedi A., Marchionni L., Agrawal N., Ravi R., Jones S., et al. Targeted sequencing reveals clonal genetic changes in the progression of early lung neoplasms and paired circulating DNA. Nat. Commun. 2015;6:8258. doi: 10.1038/ncomms9258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Macías M., Alegre E., Díaz-Lagares A., Patiño A., Pérez-Gracia J.L., Sanmamed M., López-López R., Varo N., González A. Liquid biopsy: From basic research to clinical practice. Adv. Clin. Chem. 2018;83:73–119. doi: 10.1016/bs.acc.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Freitas C., Sousa C., Machado F., Serino M., Santos V., Cruz-Martins N., Teixeira A., Cunha A., Pereira T., Oliveira H.P., et al. The role of liquid biopsy in early diagnosis of lung cancer. Front. Oncol. 2021;11:634316. doi: 10.3389/fonc.2021.634316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garrido P., Conde E., De Castro J., Gómez-Román J.J., Felip E., Pijuan L., Isla D., Sanz J., Paz-Ares L., López-Ríos F. Updated guidelines for predictive biomarker testing in advanced non-small-cell lung cancer: A National Consensus of the Spanish Society of Pathology and the Spanish Society of Medical Oncology. Clin. Transl. Oncol. 2019;22:989–1003. doi: 10.1007/s12094-019-02218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shin D.H., Shim H.S., Kim T.J., Park H.S., La Choi Y., Kim W.S., Kim L., Chang S.H., Song J.S., Kim H.J., et al. Provisional guideline recommendation for EGFR gene mutation testing in liquid samples of lung cancer patients: A proposal by the korean cardiopulmonary pathology study group. J. Pathol. Transl. Med. 2019;53:153–158. doi: 10.4132/jptm.2019.02.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corcoran R.B., Chabner B.A. Application of Cell-free DNA Analysis to Cancer Treatment. N. Engl. J. Med. 2018;379:1754–1765. doi: 10.1056/NEJMra1706174. [DOI] [PubMed] [Google Scholar]
- 12.Chen R., Xu X., Qian Z., Zhang C., Niu Y., Wang Z., Sun J., Zhang X., Yu Y. The biological functions and clinical applications of exosomes in lung cancer. Cell. Mol. Life Sci. 2019;76:4613–4633. doi: 10.1007/s00018-019-03233-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu W., Hurley J., Roberts D., Chakrabortty S., Enderle D., Noerholm M., Breakefield X., Skog J. Exosome-based liquid biopsies in cancer: Opportunities and challenges. Ann. Oncol. 2021;32:466–477. doi: 10.1016/j.annonc.2021.01.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y., Guo M., Lin D., Liang D., Zhao L., Zhao R., Wang Y. Docetaxel-loaded exosomes for targeting non-small cell lung cancer: Preparation and evaluation in vitro and in vivo. Drug Deliv. 2021;28:1510–1523. doi: 10.1080/10717544.2021.1951894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harding C., Heuser J., Stahl P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J. Cell Biol. 1983;97:329–339. doi: 10.1083/jcb.97.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pan B.T., Teng K., Wu C., Adam M., Johnstone R.M. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J. Cell Biol. 1985;101:942–948. doi: 10.1083/jcb.101.3.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colombo M., Moita C.F., van Niel G., Kowal J., Vigneron J., Benaroch P., Manel N., Moita L.F., Théry C., Raposo G. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J. Cell Sci. 2013;126:5553–5565. doi: 10.1242/jcs.128868. [DOI] [PubMed] [Google Scholar]
- 18.Balaj L., Lessard R., Dai L., Cho Y.-J., Pomeroy S.L., Breakefield X.O., Skog J. Tumour microvesicles contain retrotransposon elements and amplified oncogene sequences. Nat. Commun. 2011;2:180. doi: 10.1038/ncomms1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramteke A., Ting H., Agarwal C., Mateen S., Somasagara R., Hussain A., Graner M., Frederick B., Agarwal R., Deep G. Exosomes secreted under hypoxia enhance invasiveness and stemness of prostate cancer cells by targeting adherens junction molecules. Mol. Carcinog. 2013;54:554–565. doi: 10.1002/mc.22124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Szczepanski M.J., Szajnik M., Welsh A., Whiteside T.L., Boyiadzis M. Blast-derived microvesicles in sera from patients with acute myeloid leukemia suppress natural killer cell function via membrane-associated transforming growth factor-1. Haematologica. 2011;96:1302–1309. doi: 10.3324/haematol.2010.039743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lai C.P., Mardini O., Ericsson M., Prabhakar S., Maguire C.A., Chen J.W., Tannous B.A., Breakefield X.O. Dynamic biodistribution of extracellular vesicles in vivo using a multimodal imaging reporter. ACS Nano. 2013;8:483–494. doi: 10.1021/nn404945r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rabinowits G., Gerçel-Taylor C., Day J.M., Taylor D.D., Kloecker G.H. Exosomal MicroRNA: A diagnostic marker for lung cancer. Clin. Lung Cancer. 2009;10:42–46. doi: 10.3816/CLC.2009.n.006. [DOI] [PubMed] [Google Scholar]
- 23.Skotland T., Sandvig K., Llorente A. Lipids in exosomes: Current knowledge and the way forward. Prog. Lipid Res. 2017;66:30–41. doi: 10.1016/j.plipres.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Lai A., Elfeky O., Rice G.E., Salomon C. Optimized specific isolation of placenta-derived exosomes from maternal circulation. Preeclampsia. 2017;1710:131–138. doi: 10.1007/978-1-4939-7498-6_10. [DOI] [PubMed] [Google Scholar]
- 25.Escola J.-M., Kleijmeer M.J., Stoorvogel W., Griffith J.M., Yoshie O., Geuze H.J. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J. Biol. Chem. 1998;273:20121–20127. doi: 10.1074/jbc.273.32.20121. [DOI] [PubMed] [Google Scholar]
- 26.Theodoraki M.-N., Hoffmann T.K., Whiteside T.L. Separation of plasma-derived exosomes into CD3(+) and CD3(−) fractions allows for association of immune cell and tumour cell markers with disease activity in HNSCC patients. Clin. Exp. Immunol. 2018;192:271–283. doi: 10.1111/cei.13113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baranyai T., Herczeg K., Onódi Z., Voszka I., Módos K., Marton N., Nagy G., Mäger I., Wood M.J., El Andaloussi S., et al. Isolation of exosomes from blood plasma: Qualitative and quantitative comparison of ultracentrifugation and size exclusion chromatography methods. PLoS ONE. 2015;10:e0145686. doi: 10.1371/journal.pone.0145686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alegre E., Rebmann V., LeMaoult J., Rodriguez C., Horn P.A., Diaz-Lagares A., Echeveste J.I., González A. In vivo identification of an HLA-G complex as ubiquitinated protein circulating in exosomes. Eur. J. Immunol. 2013;43:1933–1939. doi: 10.1002/eji.201343318. [DOI] [PubMed] [Google Scholar]
- 29.Barreiro K., Dwivedi O.P., Leparc G., Rolser M., Delic D., Forsblom C., Groop P., Groop L., Huber T.B., Puhka M., et al. Comparison of urinary extracellular vesicle isolation methods for transcriptomic biomarker research in diabetic kidney disease. J. Extracell. Vesicles. 2020;10:e12038. doi: 10.1002/jev2.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campoy I., Lanau L., Altadill T., Sequeiros T., Cabrera S., Cubo-Abert M., Pérez-Benavente A., Garcia A., Borrós S., Santamaria A., et al. Exosome-like vesicles in uterine aspirates: A comparison of ultracentrifugation-based isolation protocols. J. Transl. Med. 2016;14:180. doi: 10.1186/s12967-016-0935-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tauro B.J., Greening D., Mathias R., Ji H., Mathivanan S., Scott A., Simpson R.J. Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line LIM1863-derived exosomes. Methods. 2012;56:293–304. doi: 10.1016/j.ymeth.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 32.Greening D.W., Xu R., Ji H., Tauro B.J., Simpson R.J. A protocol for exosome isolation and characterization: Evaluation of ultracentrifugation, density-gradient separation, and immunoaffinity capture methods. Methods Mol. Biol. 2015;1295:179–209. doi: 10.1007/978-1-4939-2550-6_15. [DOI] [PubMed] [Google Scholar]
- 33.Macías M., Rebmann V., Mateos B., Varo N., Perez-Gracia J.L., Alegre E., González A. Comparison of six commercial serum exosome isolation methods suitable for clinical laboratories. Effect in cytokine analysis. Clin. Chem. Lab. Med. 2019;57:1539–1545. doi: 10.1515/cclm-2018-1297. [DOI] [PubMed] [Google Scholar]
- 34.Gámez-Valero A., Monguió-Tortajada M., Carreras-Planella L., Franquesa M., Beyer K., Borràs F.E. Size-exclusion chromatography-based isolation minimally alters extracellular Vesicles’ characteristics compared to precipitating agents. Sci. Rep. 2016;6:srep33641. doi: 10.1038/srep33641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Deun J., Mestdagh P., Sormunen R., Cocquyt V., Vermaelen K., Vandesompele J., Bracke M., De Wever O., Hendrix A. The impact of disparate isolation methods for extracellular vesicles on downstream RNA profiling. J. Extracell. Vesicles. 2014;3 doi: 10.3402/jev.v3.24858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Théry C., Amigorena S., Raposo G., Clayton A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 2006;30:3–22. doi: 10.1002/0471143030.cb0322s30. [DOI] [PubMed] [Google Scholar]
- 37.Livshits M.A., Khomyakova E., Evtushenko E., Lazarev V.N., Kulemin N., Semina S.E., Generozov E., Govorun V.M. Isolation of exosomes by differential centrifugation: Theoretical analysis of a commonly used protocol. Sci. Rep. 2015;5:17319. doi: 10.1038/srep17319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cvjetkovic A., Lötvall J., Lässer C. The influence of rotor type and centrifugation time on the yield and purity of extracellular vesicles. J. Extracell. Vesicles. 2014;3:23111. doi: 10.3402/jev.v3.23111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Witwer K.W., Buzás E.I., Bemis L., Bora A., Lässer C., Lötvall J., Hoen E.N.N., Piper M.G., Sivaraman S., Skog J., et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J. Extracell. Vesicles. 2013;2:20360. doi: 10.3402/jev.v2i0.20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jeppesen D., Hvam M.L., Primdahl-Bengtson B., Boysen A.T., Whitehead B., Dyrskjøt L., Ørntoft T.F., Howard K.A., Ostenfeld M.S. Comparative analysis of discrete exosome fractions obtained by differential centrifugation. J. Extracell. Vesicles. 2014;3:25011. doi: 10.3402/jev.v3.25011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Momen-Heravi F., Balaj L., Alian S., Trachtenberg A.J., Hochberg F.H., Skog J., Kuo W.P. Impact of biofluid viscosity on size and sedimentation efficiency of the isolated microvesicles. Front. Physiol. 2012;3:162. doi: 10.3389/fphys.2012.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Z., Wang C., Li T., Liu Z., Li L. Comparison of ultracentrifugation and density gradient separation methods for isolating Tca8113 human tongue cancer cell line-derived exosomes. Oncol. Lett. 2014;8:1701–1706. doi: 10.3892/ol.2014.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lobb R., Becker M., Wen S.W., Wong C.S.F., Wiegmans A.P., Leimgruber A., Möller A. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J. Extracell. Vesicles. 2015;4:27031. doi: 10.3402/jev.v4.27031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nordin J., Lee Y., Vader P., Mäger I., Johansson H., Heusermann W., Wiklander O.P., Hällbrink M., Seow Y., Bultema J.J., et al. Ultrafiltration with size-exclusion liquid chromatography for high yield isolation of extracellular vesicles preserving intact biophysical and functional properties. Nanomed. Nanotechnol. Biol. Med. 2015;11:879–883. doi: 10.1016/j.nano.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 45.Zarovni N., Corrado A., Guazzi P., Zocco D., Lari E., Radano G., Muhhina J., Fondelli C., Gavrilova J., Chiesi A. Integrated isolation and quantitative analysis of exosome shuttled proteins and nucleic acids using immunocapture approaches. Methods. 2015;87:46–58. doi: 10.1016/j.ymeth.2015.05.028. [DOI] [PubMed] [Google Scholar]
- 46.Wubbolts R., Leckie R.S., Veenhuizen P.T.M., Schwarzmann G., Möbius W., Hoernschemeyer J., Slot J.-W., Geuze H.J., Stoorvogel W. Proteomic and biochemical analyses of human B cell-derived exosomes. J. Biol. Chem. 2003;278:10963–10972. doi: 10.1074/jbc.M207550200. [DOI] [PubMed] [Google Scholar]
- 47.Popović M., de Marco A. Canonical and selective approaches in exosome purification and their implications for diagnostic accuracy. Transl. Cancer Res. 2017;7:S209–S225. doi: 10.21037/tcr.2017.08.44. [DOI] [Google Scholar]
- 48.Serrano-Pertierra E., Oliveira-Rodríguez M., Rivas M., Oliva P., Villafani J., Navarro A., Blanco-López M.C., Cernuda-Morollón E. Characterization of plasma-derived extracellular vesicles isolated by different methods: A comparison study. Bioengineering. 2019;6:8. doi: 10.3390/bioengineering6010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brownlee Z., Lynn K.D., Thorpe P.E., Schroit A.J. A novel “salting-out” procedure for the isolation of tumor-derived exosomes. J. Immunol. Methods. 2014;407:120–126. doi: 10.1016/j.jim.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim Y.B., Yang J.S., Bin Lee G., Moon M.H. Evaluation of exosome separation from human serum by frit-inlet asymmetrical flow field-flow fractionation and multiangle light scattering. Anal. Chim. Acta. 2020;1124:137–145. doi: 10.1016/j.aca.2020.05.031. [DOI] [PubMed] [Google Scholar]
- 51.Alvarez P.V., Blazquez R., Sánchez M.F., DelaRosa O., Jorge I., Tapia-Araya A., Casado J. Comparative study of isolated human mesenchymal stem cell derived exosomes for clinical use. Acta Bioquím. Clín. Latinoam. 2015;49:311–320. [Google Scholar]
- 52.Diaz G., Bridges C., Lucas M., Cheng Y., Schorey J.S., Dobos K.M., Kruh-Garcia N.A. Protein digestion, ultrafiltration, and size exclusion chromatography to optimize the isolation of exosomes from human blood plasma and serum. J. Vis. Exp. 2018:e57467. doi: 10.3791/57467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kooijmans S.A.A., Aleza C.G., Roffler S.R., Van Solinge W., Vader P., Schiffelers R.M. Display of GPI-anchored anti-EGFR nanobodies on extracellular vesicles promotes tumour cell targeting. J. Extracell. Vesicles. 2016;5:31053. doi: 10.3402/jev.v5.31053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Heinemann M., Ilmer M., Silva L.P., Hawke D., Recio A., Vorontsova M.A., Alt E., Vykoukal J. Benchtop isolation and characterization of functional exosomes by sequential filtration. J. Chromatogr. A. 2014;1371:125–135. doi: 10.1016/j.chroma.2014.10.026. [DOI] [PubMed] [Google Scholar]
- 55.Heinemann M.L., Vykoukal J. Sequential filtration: A gentle method for the isolation of functional extracellular vesicles. In: Kuo W.P., Jia S., editors. Extracellular Vesicles: Methods and Protocols. Springer; New York, NY, USA: 2017. pp. 33–41. [DOI] [PubMed] [Google Scholar]
- 56.Liu C., Guoqing H., Tian F., Yang N., Yanping D., Ding Y., Wei J., Hu G., Nie G., Sun J. Field-free isolation of exosomes from extracellular vesicles by microfluidic viscoelastic flows. ACS Nano. 2017;11:6968–6976. doi: 10.1021/acsnano.7b02277. [DOI] [PubMed] [Google Scholar]
- 57.Musante L., Tataruch D., Gu D., Martin A.B., Calzaferri G., Aherne S., Holthofer H. A simplified method to recover urinary vesicles for clinical applications and sample banking. Sci. Rep. 2014;4:7532. doi: 10.1038/srep07532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu Y., Qin S., An T., Tang Y., Huang Y., Zheng L. MiR-145 detection in urinary extracellular vesicles increase diagnostic efficiency of prostate cancer based on hydrostatic filtration dialysis method. Prostate. 2017;77:1167–1175. doi: 10.1002/pros.23376. [DOI] [PubMed] [Google Scholar]
- 59.Vogel R., Coumans F.A.W., Maltesen R., Böing A.N., Bonnington K.E., Broekman M.L., Broom M.F., Buzás E.I., Christiansen G., Hajji N., et al. A standardized method to determine the concentration of extracellular vesicles using tunable resistive pulse sensing. J. Extracell. Vesicles. 2016;5:31242. doi: 10.3402/jev.v5.31242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Böing A.N., van der Pol E., Grootemaat A.E., Coumans F.A.W., Sturk A., Nieuwland R. Single-step isolation of extracellular vesicles by size-exclusion chromatography. J. Extracell. Vesicles. 2014;3:23430. doi: 10.3402/jev.v3.23430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lozano-Ramos I., Bancu I., Oliveira-Tercero A., Armengol M.P., Menezes-Neto A., Del Portillo H.A., Lauzurica-Valdemoros R., Borràs F.E. Size-exclusion chromatography-based enrichment of extracellular vesicles from urine samples. J. Extracell. Vesicles. 2015;4:27369. doi: 10.3402/jev.v4.27369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Giddings J.C., Yang F.J., Myers M.N. Flow-field-flow fractionation: A versatile new separation method. Science. 1976;193:1244–1245. doi: 10.1126/science.959835. [DOI] [PubMed] [Google Scholar]
- 63.Zhang H., Freitas D., Kim H.S., Fabijanic K., Li Z., Chen H., Mark M.T., Molina H., Martin A.B., Bojmar L., et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nature. 2018;20:332–343. doi: 10.1038/s41556-018-0040-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Clayton A., Court J., Navabi H., Adams M., Mason M.D., Hobot J.A., Newman G.R., Jasani B. Analysis of antigen presenting cell derived exosomes, based on immuno-magnetic isolation and flow cytometry. J. Immunol. Methods. 2001;247:163–174. doi: 10.1016/S0022-1759(00)00321-5. [DOI] [PubMed] [Google Scholar]
- 65.Deregibus M.C., Figliolini F., D′Antico S., Manzini P.M., Pasquino C., De Lena M., Tetta C., Brizzi M.F., Camussi G. Charge-based precipitation of extracellular vesicles. Int. J. Mol. Med. 2016;38:1359–1366. doi: 10.3892/ijmm.2016.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.He M., Crow J., Roth M., Zeng Y., Godwin A.K. Integrated immunoisolation and protein analysis of circulating exosomes using microfluidic technology. Lab Chip. 2014;14:3773–3780. doi: 10.1039/C4LC00662C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kanwar S.S., Dunlay C.J., Simeone D.M., Nagrath S. Microfluidic device (ExoChip) for on-chip isolation, quantification and characterization of circulating exosomes. Lab Chip. 2014;14:1891–1900. doi: 10.1039/C4LC00136B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Santana S.M., Antonyak M.A., Cerione R.A., Kirby B.J. Microfluidic isolation of cancer-cell-derived microvesicles from hetergeneous extracellular shed vesicle populations. Biomed. Microdevices. 2014;16:869–877. doi: 10.1007/s10544-014-9891-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dudani J., Gossett D.R., Tse H.T.K., Lamm R.J., Kulkarni R.P., Di Carlo D. Rapid inertial solution exchange for enrichment and flow cytometric detection of microvesicles. Biomicrofluidics. 2015;9:014112. doi: 10.1063/1.4907807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Davies R.T., Kim J., Jang S.C., Choi E.-J., Gho Y.S., Park J. Microfluidic filtration system to isolate extracellular vesicles from blood. Lab Chip. 2012;12:5202–5210. doi: 10.1039/c2lc41006k. [DOI] [PubMed] [Google Scholar]
- 71.Zhao Z., Yang Y., Zeng Y., He M. A microfluidic ExoSearch chip for multiplexed exosome detection towards blood-based ovarian cancer diagnosis. Lab Chip. 2015;16:489–496. doi: 10.1039/C5LC01117E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Royo F., Théry C., Falcón-Pérez J.M., Nieuwland R., Witwer K.W. Methods for separation and characterization of extracellular vesicles: Results of a worldwide survey performed by the ISEV rigor and standardization subcommittee. Cells. 2020;9:1955. doi: 10.3390/cells9091955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zaborowski M.P., Lee K., Na Y.J., Sammarco A., Zhang X., Iwanicki M., Cheah P.S., Lin H.-Y., Zinter M., Chou C.-Y., et al. Methods for systematic identification of membrane proteins for specific capture of cancer-derived extracellular vesicles. Cell Rep. 2019;27:255–268.e6. doi: 10.1016/j.celrep.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Choi E.-S., Faruque H., Kim J.-H., Kim K., Choi J., Kim B., Kim B., Kim Y., Woo M., Park J., et al. CD5L as an extracellular vesicle-derived biomarker for liquid biopsy of lung cancer. Diagnostics. 2021;11:620. doi: 10.3390/diagnostics11040620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yuana Y., Koning R., Kuil M.E., Rensen P.C.N., Koster A., Bertina R.M., Osanto S. Cryo-electron microscopy of extracellular vesicles in fresh plasma. J. Extracell. Vesicles. 2013;2:21494. doi: 10.3402/jev.v2i0.21494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ashby J., Flack K., Jimenez L.A., Duan Y., Khatib A.-K., Somlo G., Wang S.E., Cui X., Zhong W. Distribution profiling of circulating MicroRNAs in serum. Anal. Chem. 2014;86:9343–9349. doi: 10.1021/ac5028929. [DOI] [PubMed] [Google Scholar]
- 77.Gerratana L., Basile D., Toffoletto B., Bulfoni M., Zago S., Magini A., Lera M., Pelizzari G., Parisse P., Casalis L., et al. Biologically driven cut-off definition of lymphocyte ratios in metastatic breast cancer and association with exosomal subpopulations and prognosis. Sci. Rep. 2020;10:7010. doi: 10.1038/s41598-020-63291-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pan D., Chen J., Feng C., Wu W., Wang Y., Tong J., Zhou D. Preferential localization of MUC1 glycoprotein in exosomes secreted by non-small cell lung carcinoma cells. Int. J. Mol. Sci. 2019;20:323. doi: 10.3390/ijms20020323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yoh K.E., Lowe C.J., Mahajan S., Suttmann R., Nguy T., Reichelt M., Yang J., Melendez R., Li Y., Molinero L., et al. Enrichment of circulating tumor-derived extracellular vesicles from human plasma. J. Immunol. Methods. 2020;490:112936. doi: 10.1016/j.jim.2020.112936. [DOI] [PubMed] [Google Scholar]
- 80.Théry C., Witwer K.W., Aikawa E., Alcaraz M.J., Anderson J.D., Andriantsitohaina R., Antoniou A., Arab T., Archer F., Atkin-Smith G.K., et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles. 2018;7:1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lötvall J., Hill A., Hochberg F., Buzás E.I., Di Vizio D., Gardiner C., Gho Y.S., Kurochkin I.V., Mathivanan S., Quesenberry P., et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: A position statement from the International Society for Extracellular Vesicles. J. Extracell. Vesicles. 2014;3:26913. doi: 10.3402/jev.v3.26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Alegre E., Zubiri L., Perez-Gracia J.L., González-Cao M., Soria L., Martín-Algarra S., González A. Circulating melanoma exosomes as diagnostic and prognosis biomarkers. Clin. Chim. Acta. 2016;454:28–32. doi: 10.1016/j.cca.2015.12.031. [DOI] [PubMed] [Google Scholar]
- 83.Helwa I., Cai J., Drewry M.D., Zimmerman A., Dinkins M.B., Khaled M.L., Seremwe M., Dismuke W.M., Bieberich E., Stamer W.D., et al. A comparative study of serum exosome isolation using differential ultracentrifugation and three commercial reagents. PLoS ONE. 2017;12:e0170628. doi: 10.1371/journal.pone.0170628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jakobsen K.R., Paulsen B.S., Bæk R., Varming K., Sorensen B., Jørgensen M.M. Exosomal proteins as potential diagnostic markers in advanced non-small cell lung carcinoma. J. Extracell. Vesicles. 2015;4:26659. doi: 10.3402/jev.v4.26659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Poupardin R., Wolf M., Strunk D. Adherence to minimal experimental requirements for defining extracellular vesicles and their functions. Adv. Drug Deliv. Rev. 2021;176:113872. doi: 10.1016/j.addr.2021.113872. [DOI] [PubMed] [Google Scholar]
- 86.Mulcahy L., Pink R., Carter D.R.F. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles. 2014;3 doi: 10.3402/jev.v3.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Valadi H., Ekström K., Bossios A., Sjöstrand M., Lee J.J., Lötvall J. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 88.Fabbri M., Paone A., Calore F., Galli R., Gaudio E., Santhanam R., Lovat F., Fadda P., Mao C., Nuovo G.J., et al. MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response. Proc. Natl. Acad. Sci. USA. 2012;109:E2110–E2116. doi: 10.1073/pnas.1209414109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang F., Yan Y., Yang Y., Hong X., Wang M., Yang Z., Liu B., Ye L. MiR-210 in exosomes derived from CAFs promotes non-small cell lung cancer migration and invasion through PTEN/PI3K/AKT pathway. Cell. Signal. 2020;73:109675. doi: 10.1016/j.cellsig.2020.109675. [DOI] [PubMed] [Google Scholar]
- 90.Zhang R., Xia Y., Wang Z., Zheng J., Chen Y., Li X., Wang Y., Ming H. Serum long non coding RNA MALAT-1 protected by exosomes is up-regulated and promotes cell proliferation and migration in non-small cell lung cancer. Biochem. Biophys. Res. Commun. 2017;490:406–414. doi: 10.1016/j.bbrc.2017.06.055. [DOI] [PubMed] [Google Scholar]
- 91.Taverna S., Pucci M., Giallombardo M., Di Bella M.A., Santarpia M., Reclusa P., Gil-Bazo I., Rolfo C., Alessandro R. Amphiregulin contained in NSCLC-exosomes induces osteoclast differentiation through the activation of EGFR pathway. Sci. Rep. 2017;7:3170. doi: 10.1038/s41598-017-03460-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chalmin F., Ladoire S., Mignot G., Vincent J., Bruchard M., Remy-Martin J.-P., Boireau W., Rouleau A., Simon B., Lanneau D., et al. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J. Clin. Investig. 2010;120:457–471. doi: 10.1172/JCI40483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Whiteside T.L. Tumor-derived exosomes and their role in cancer progression. J. Clin. Investig. 2016;74:103–141. doi: 10.1016/bs.acc.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liu Y., Luo F., Wang B., Li H., Xu Y., Liu X., Shi L., Lu X., Xu W., Lu L., et al. STAT3-regulated exosomal miR-21 promotes angiogenesis and is involved in neoplastic processes of transformed human bronchial epithelial cells. Cancer Lett. 2016;370:125–135. doi: 10.1016/j.canlet.2015.10.011. [DOI] [PubMed] [Google Scholar]
- 95.Wu H., Zhou J., Mei S., Wu D., Mu Z., Chen B., Xie Y., Ye Y., Liu J. Circulating exosomal microRNA-96 promotes cell proliferation, migration and drug resistance by targeting LMO7. J. Cell. Mol. Med. 2016;21:1228–1236. doi: 10.1111/jcmm.13056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang Y., Li M., Hu C. Exosomal transfer of miR-214 mediates gefitinib resistance in non-small cell lung cancer. Biochem. Biophys. Res. Commun. 2018;507:457–464. doi: 10.1016/j.bbrc.2018.11.061. [DOI] [PubMed] [Google Scholar]
- 97.Wu S., Luo M., To K.K.W., Zhang J., Su C., Zhang H., An S., Wang F., Chen D., Fu L. Intercellular transfer of exosomal wild type EGFR triggers osimertinib resistance in non-small cell lung cancer. Mol. Cancer. 2021;20:1–17. doi: 10.1186/s12943-021-01307-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brabletz T., Kalluri R., Nieto M.A., Weinberg R.A. EMT in cancer. Nat. Rev. Cancer. 2018;18:128–134. doi: 10.1038/nrc.2017.118. [DOI] [PubMed] [Google Scholar]
- 99.Rahman M.A., Barger J.F., Lovat F., Gao M., Otterson G.A., Nana-Sinkam P. Lung cancer exosomes as drivers of epithelial mesenchymal transition. Oncotarget. 2016;7:54852–54866. doi: 10.18632/oncotarget.10243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.He S., Li Z., Yu Y., Zeng Q., Cheng Y., Ji W., Xia W., Lu S. Exosomal miR-499a-5p promotes cell proliferation, migration and EMT via mTOR signaling pathway in lung adenocarcinoma. Exp. Cell Res. 2019;379:203–213. doi: 10.1016/j.yexcr.2019.03.035. [DOI] [PubMed] [Google Scholar]
- 101.Kim J., Kim T.Y., Lee M.S., Mun J.Y., Ihm C., Kim S.A. Exosome cargo reflects TGF-β1-mediated epithelial-to-mesenchymal transition (EMT) status in A549 human lung adenocarcinoma cells. Biochem. Biophys. Res. Commun. 2016;478:643–648. doi: 10.1016/j.bbrc.2016.07.124. [DOI] [PubMed] [Google Scholar]
- 102.Hoshino A., Costa-Silva B., Shen T.-L., Rodrigues G., Hashimoto A., Mark M.T., Molina H., Kohsaka S., Di Giannatale A., Ceder S., et al. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527:329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Du C., Duan X., Yao X., Wan J., Cheng Y., Wang Y., Yan Y., Zhang L., Zhu L., Ni C., et al. Tumour-derived exosomal miR-3473b promotes lung tumour cell intrapulmonary colonization by activating the nuclear factor-κB of local fibroblasts. J. Cell. Mol. Med. 2020;24:7802–7813. doi: 10.1111/jcmm.15411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gan D.-X., Wang Y.-B., He M.-Y., Chen Z.-Y., Qin X.-X., Miao Z.-W., Chen Y.-H., Li B. Lung cancer cells-controlled Dkk-1 production in brain metastatic cascade drive microglia to acquire a pro-tumorigenic phenotype. Front. Cell Dev. Biol. 2020;8:1594. doi: 10.3389/fcell.2020.591405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hsu Y.-L., Hung J.-Y., Chang W.-A., Lin Y.-S., Pan Y.-C., Tsai P.-H., Wu C.-Y., Kuo P.-L. Hypoxic lung cancer-secreted exosomal miR-23a increased angiogenesis and vascular permeability by targeting prolyl hydroxylase and tight junction protein ZO-1. Oncogene. 2017;36:4929–4942. doi: 10.1038/onc.2017.105. [DOI] [PubMed] [Google Scholar]
- 106.Li Z., Zeng C., Nong Q., Long F., Liu J., Mu Z., Chen B., Wu D., Wu H. Exosomal leucine-rich-alpha2-glycoprotein 1 Derived from Non-Small-Cell Lung Cancer Cells Promotes angiogenesis via TGF-β signal pathway. Mol. Ther.-Oncolytics. 2019;14:313–322. doi: 10.1016/j.omto.2019.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Huang S.-H., Li Y., Zhang J., Rong J., Ye S. Epidermal growth factor receptor-containing exosomes induce tumor-specific regulatory T cells. Cancer Investig. 2013;31:330–335. doi: 10.3109/07357907.2013.789905. [DOI] [PubMed] [Google Scholar]
- 108.Berchem G., Noman M.Z., Bosseler M., Paggetti J., Baconnais S., Le Cam E., Nanbakhsh A., Moussay E., Mami-Chouaib F., Janji B., et al. Hypoxic tumor-derived microvesicles negatively regulate NK cell function by a mechanism involving TGF-β and miR23a transfer. OncoImmunology. 2015;5:e1062968. doi: 10.1080/2162402X.2015.1062968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chen G., Huang A.C., Zhang W., Zhang G., Wu M., Xu W., Yu Z., Yang J., Wang B., Sun H., et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560:382–386. doi: 10.1038/s41586-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wu J., Shen Z. Exosomal miRNAs as biomarkers for diagnostic and prognostic in lung cancer. Cancer Med. 2020;9:6909–6922. doi: 10.1002/cam4.3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sandfeld-Paulsen B., Jakobsen K.R., Bæk R., Folkersen B.H., Rasmussen T.R., Meldgaard P., Varming K., Jørgensen M.M., Sorensen B. Exosomal proteins as diagnostic biomarkers in lung cancer. J. Thorac. Oncol. 2016;11:1701–1710. doi: 10.1016/j.jtho.2016.05.034. [DOI] [PubMed] [Google Scholar]
- 112.Ueda K., Ishikawa N., Tatsuguchi A., Saichi N., Fujii R., Nakagawa H. Antibody-coupled monolithic silica microtips for highthroughput molecular profiling of circulating exosomes. Sci. Rep. 2014;4:srep06232. doi: 10.1038/srep06232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Niu L., Song X., Wang N., Xue L., Song X., Xie L. Tumor-derived exosomal proteins as diagnostic biomarkers in non-small cell lung cancer. Cancer Sci. 2018;110:433–442. doi: 10.1111/cas.13862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Vykoukal J., Sun N., Aguilar-Bonavides C., Katayama H., Tanaka I., Fahrmann J.F., Capello M., Fujimoto J., Aguilar M., Wistuba I.I., et al. Plasma-derived extracellular vesicle proteins as a source of biomarkers for lung adenocarcinoma. Oncotarget. 2017;8:95466–95480. doi: 10.18632/oncotarget.20748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Li Y., Zhang Y., Qiu F., Qiu Z. Proteomic identification of exosomal LRG1: A potential urinary biomarker for detecting NSCLC. Electrophoresis. 2011;32:1976–1983. doi: 10.1002/elps.201000598. [DOI] [PubMed] [Google Scholar]
- 116.Sandfeld-Paulsen B., Aggerholm-Pedersen N., Baek R., Jakobsen K.R., Meldgaard P., Folkersen B., Rasmussen T.R., Varming K., Jørgensen M.M., Sorensen B. Exosomal proteins as prognostic biomarkers in non-small cell lung cancer. Mol. Oncol. 2016;10:1595–1602. doi: 10.1016/j.molonc.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yang Q., Chen M., Gu J., Niu K., Zhao X., Zheng L., Xu Z., Yu Y., Li F., Meng L., et al. Novel biomarkers of dynamic blood PD-L1 expression for immune checkpoint inhibitors in advanced non-small-cell lung cancer patients. Front. Immunol. 2021;12:665133. doi: 10.3389/fimmu.2021.665133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chanteloup G., Cordonnier M., Isambert N., Bertaut A., Hervieu A., Hennequin A., Luu M., Zanetta S., Coudert B., Bengrine L., et al. Monitoring HSP70 exosomes in cancer patients’ follow up: A clinical prospective pilot study. J. Extracell. Vesicles. 2020;9:1766192. doi: 10.1080/20013078.2020.1766192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cazzoli R., Buttitta F., DI Nicola M., Malatesta S., Marchetti A., Rom W., Pass H.I. microRNAs derived from circulating exosomes as noninvasive biomarkers for screening and diagnosing lung cancer. J. Thorac. Oncol. 2013;8:1156–1162. doi: 10.1097/JTO.0b013e318299ac32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang X., Jiang X., Li J., Wang J., Binang H., Shi S., Duan W., Zhao Y., Zhang Y. Serum exosomal miR-1269a serves as a diagnostic marker and plays an oncogenic role in non-small cell lung cancer. Thorac. Cancer. 2020;11:3436–3447. doi: 10.1111/1759-7714.13644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang Z., Tang Y., Song X., Xie L., Zhao S., Song X. Tumor-derived exosomal miRNAs as diagnostic biomarkers in non-small cell lung cancer. Front. Oncol. 2020;10:560025. doi: 10.3389/fonc.2020.560025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang J., Xue H., Zhu Z., Gao J., Zhao M., Ma Z. Expression of serum exosomal miR-23b-3p in non-small cell lung cancer and its diagnostic efficacy. Oncol. Lett. 2020;20:30. doi: 10.3892/ol.2020.11891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wang N., Guo W., Song X., Liu L., Niu L., Song X., Xie L. Tumor-associated exosomal miRNA biomarkers to differentiate metastatic vs. nonmetastatic non-small cell lung cancer. Clin. Chem. Lab. Med. 2020;58:1535–1545. doi: 10.1515/cclm-2019-1329. [DOI] [PubMed] [Google Scholar]
- 124.Zhang Z.-J., Song X.-G., Xie L., Wang K.-Y., Tang Y.-Y., Yu M., Feng X.-D., Song X.-R. Circulating serum exosomal miR-20b-5p and miR-3187-5p as efficient diagnostic biomarkers for early-stage non-small cell lung cancer. Exp. Biol. Med. 2020;245:1428–1436. doi: 10.1177/1535370220945987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yang G., Wang T., Qu X., Chen S., Han Z., Chen S., Chen M., Lin J., Yu S., Gao L., et al. Exosomal miR-21/Let-7a ratio distinguishes non-small cell lung cancer from benign pulmonary diseases. Asia-Pac. J. Clin. Oncol. 2020;16:280–286. doi: 10.1111/ajco.13343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jin X., Chen Y., Chen H., Fei S., Chen D., Cai X., Liu L., Lin B., Su H., Zhao L., et al. Evaluation of tumor-derived exosomal miRNA as potential diagnostic biomarkers for early-stage non–small cell lung cancer using next-generation sequencing. Clin. Cancer Res. 2017;23:5311–5319. doi: 10.1158/1078-0432.CCR-17-0577. [DOI] [PubMed] [Google Scholar]
- 127.Dejima H., Iinuma H., Kanaoka R., Matsutani N., Kawamura M. Exosomal microRNA in plasma as a non-invasive biomarker for the recurrence of non-small cell lung cancer. Oncol. Lett. 2017;13:1256–1263. doi: 10.3892/ol.2017.5569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lin J., Wang Y., Zou Y.-Q., Chen X., Huang B., Liu J., Xu Y.-M., Zhang J., Yang W.-M., Wei-Ming Y., et al. Differential miRNA expression in pleural effusions derived from extracellular vesicles of patients with lung cancer, pulmonary tuberculosis, or pneumonia. Tumor Biol. 2016;37:15835–15845. doi: 10.1007/s13277-016-5410-6. [DOI] [PubMed] [Google Scholar]
- 129.Halvorsen A.R., Bjaanæs M., Leblanc M., Holm A.M., Bolstad N., Rubio L., Peñalver J.C., Cervera J., Mojarrieta J.C., López-Guerrero J.A., et al. A unique set of 6 circulating microRNAs for early detection of non-small cell lung cancer. Oncotarget. 2016;7:37250–37259. doi: 10.18632/oncotarget.9363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tamiya H., Mitani A., Saito A., Ishimori T., Saito M., Isago H., Jo T., Yamauchi Y., Tanaka G., Nagase T. Exosomal microRNA expression profiling in patients with lung adenocarcinoma-associated malignant pleural effusion. Anticancer Res. 2018;38:6707–6714. doi: 10.21873/anticanres.13039. [DOI] [PubMed] [Google Scholar]
- 131.Zhou P., Lu F., Wang J., Wang K., Liu B., Li N., Tang B. A portable point-of-care testing system to diagnose lung cancer through the detection of exosomal miRNA in urine and saliva. Chem. Commun. 2020;56:8968–8971. doi: 10.1039/D0CC03180A. [DOI] [PubMed] [Google Scholar]
- 132.Yang X.-R., Pi C., Yu R., Fan X.-J., Peng X.-X., Zhang X.-C., Chen Z.-H., Wu X., Shao Y., Wu Y.-L., et al. Correlation of exosomal microRNA clusters with bone metastasis in non-small cell lung cancer. Clin. Exp. Metastasis. 2020;38:109–117. doi: 10.1007/s10585-020-10062-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yuwen D.-L., Sheng B.-B., Liu J., Wenyu W., Shu Y.-Q. MiR-146a-5p level in serum exosomes predicts therapeutic effect of cisplatin in non-small cell lung cancer. Eur. Rev. Med. Pharmacol. Sci. 2017;21:2650–2658. [PubMed] [Google Scholar]
- 134.Zheng Q., Ding H., Wang L., Yan Y., Wan Y., Yi Y., Tao L., Zhu C. Circulating exosomal miR-96 as a novel biomarker for radioresistant non-small-cell lung cancer. J. Oncol. 2021;2021:5893981. doi: 10.1155/2021/5893981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Peng X.X., Yu R., Wu X., Wu S.Y., Pi C., Chen Z.H., Zhang X.C., Gao C.Y., Shao Y.W., Liu L., et al. Correlation of plasma exosomal microRNAs with the efficacy of immunotherapy in EGFR/ALK wild-type advanced non-small cell lung cancer. J. Immunother. Cancer. 2020;8:e000376. doi: 10.1136/jitc-2019-000376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Cao B., Wang P., Gu L., Liu J. Use of four genes in exosomes as biomarkers for the identification of lung adenocarcinoma and lung squamous cell carcinoma. Oncol. Lett. 2021;21:249. doi: 10.3892/ol.2021.12510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dong Q., Dong L., Liu S., Kong Y., Zhang M., Wang X. Tumor-derived exosomal eIF4E as a biomarker for survival prediction in patients with non-small cell lung cancer. Med. Sci. Monit. 2020;26:e923210. doi: 10.12659/MSM.923210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Del Re M., Cucchiara F., Rofi E., Fontanelli L., Petrini I., Gri N., Pasquini G., Rizzo M., Gabelloni M., Belluomini L., et al. A multiparametric approach to improve the prediction of response to immunotherapy in patients with metastatic NSCLC. Cancer Immunol. Immunother. 2020;70:1667–1678. doi: 10.1007/s00262-020-02810-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Xian J., Zeng Y., Chen S., Lu L., Liu L., Chen J., Rao B., Zhao Z., Liu J., Xie C., et al. Discovery of a novel linc01125 isoform in serum exosomes as a promising biomarker for NSCLC diagnosis and survival assessment. Carcinogenesis. 2021;42:831–841. doi: 10.1093/carcin/bgab034. [DOI] [PubMed] [Google Scholar]
- 140.Li L., Li W., Chen N., Zhao H., Xu G., Zhao Y., Pan X., Zhang X., Zhou L., Yu D., et al. FLI1 exonic circular RNAs as a novel oncogenic driver to promote tumor metastasis in small cell lung cancer. Clin. Cancer Res. 2018;25:1302–1317. doi: 10.1158/1078-0432.CCR-18-1447. [DOI] [PubMed] [Google Scholar]
- 141.Wang Y., Zhang H., Wang J., Li B., Wang X. Circular RNA expression profile of lung squamous cell carcinoma: Identification of potential biomarkers and therapeutic targets. Biosci. Rep. 2020;40:BSR20194512. doi: 10.1042/BSR20194512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.He F., Zhong X., Lin Z., Lin J., Qiu M., Li X., Hu Z. Plasma exo-hsa_circRNA_0056616: A potential biomarker for lymph node metastasis in lung adenocarcinoma. J. Cancer. 2020;11:4037–4046. doi: 10.7150/jca.30360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhang N., Nan A., Chen L., Li X., Jia Y., Qiu M., Dai X., Zhou H., Zhu J., Zhang H., et al. Circular RNA circSATB2 promotes progression of non-small cell lung cancer cells. Mol. Cancer. 2020;19:101. doi: 10.1186/s12943-020-01221-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Xian J., Su W., Liu L., Rao B., Lin M., Feng Y., Qiu F., Chen J., Zhou Q., Zhao Z., et al. Identification of three circular RNA cargoes in serum exosomes as diagnostic biomarkers of non–small-cell lung cancer in the chinese population. J. Mol. Diagn. 2020;22:1096–1108. doi: 10.1016/j.jmoldx.2020.05.011. [DOI] [PubMed] [Google Scholar]
- 145.Jørgensen M.M., Baek R., Pedersen S., Sondergaard E.K.L., Kristensen S.R., Varming K. Extracellular Vesicle (EV) Array: Microarray capturing of exosomes and other extracellular vesicles for multiplexed phenotyping. J. Extracell. Vesicles. 2013;2:20920. doi: 10.3402/jev.v2i0.20920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Erdbrügger U., Blijdorp C.J., Bijnsdorp I.V., Borràs F.E., Burger D., Bussolati B., Byrd J.B., Clayton A., Dear J.W., Falcón-Pérez J.M., et al. Urinary extracellular vesicles: A position paper by the Urine Task Force of the International Society for Extracellular Vesicles. J. Extracell. Vesicles. 2021;10:e12093. doi: 10.1002/jev2.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Furukawa K., Kawamoto K., Eguchi H., Tanemura M., Tanida T., Tomimaru Y., Akita H., Hama N., Wada H., Kobayashi S., et al. Clinicopathological significance of leucine-rich α2-glycoprotein-1 in sera of patients with pancreatic cancer. Pancreas. 2015;44:93–98. doi: 10.1097/MPA.0000000000000205. [DOI] [PubMed] [Google Scholar]
- 148.Wu J., Yin H., Zhu J., Buckanovich R.J., Thorpe J.D., Dai J., Urban N., Lubman D.M. Validation of LRG1 as a potential biomarker for detection of epithelial ovarian cancer by a blinded study. PLoS ONE. 2015;10:e0121112. doi: 10.1371/journal.pone.0121112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Alegre E., Fusco J.P., Restituto P., Salas D., Rodriguez-Ruiz E.M., Andueza M.-P., Pajares M.J., Patiño-García A., Pio R., Lozano M.D., et al. Total and mutated EGFR quantification in cell-free DNA from non-small cell lung cancer patients detects tumor heterogeneity and presents prognostic value. Tumor Biol. 2016;37:13687–13694. doi: 10.1007/s13277-016-5282-9. [DOI] [PubMed] [Google Scholar]
- 150.Fortunato O., Gasparini P., Boeri M., Sozzi G. Exo-miRNAs as a new tool for liquid biopsy in lung cancer. Cancers. 2019;11:888. doi: 10.3390/cancers11060888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kuchuk M., Addison C.L., Clemons M., Kuchuk I., Wheatley-Price P. Incidence and consequences of bone metastases in lung cancer patients. J. Bone Oncol. 2013;2:22–29. doi: 10.1016/j.jbo.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Brinkmann K., Enderle D., Koestler T., Bentink S., Emenegger J., Spiel A., Mueller R., O′Neill V., Skog J., Noerholm M. Abstract 545: Plasma-based diagnostics for detection of EML4-ALK fusion transcripts in NSCLC patients. Cancer Res. 2015;75:545. doi: 10.1158/1538-7445.am2015-545. [DOI] [Google Scholar]
- 153.Karimzadeh M.R., Seyedtaghia M.R., Soudyab M., Nezamnia M., Kidde J., Sahebkar A. Exosomal long noncoding RNAs: Insights into emerging diagnostic and therapeutic applications in lung cancer. J. Oncol. 2020;2020:7630197. doi: 10.1155/2020/7630197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wu Z., Xu Z., Yu B., Zhang J., Yu B. The Potential Diagnostic Value of Exosomal Long Noncoding RNAs in Solid Tumors: A Meta-Analysis and Systematic Review. BioMed Res. Int. 2020;2020:6786875. doi: 10.1155/2020/6786875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Macías M., Alegre E., Alkorta-Aranburu G., Patiño-García A., Mateos B., Andueza M.P., Gúrpide A., Lopez-Picazo J.M., Gil-Bazo I., Perez-Gracia J.L., et al. The dynamic use of EGFR mutation analysis in cell-free DNA as a follow-up biomarker during different treatment lines in non-small-cell lung cancer patients. Dis. Markers. 2019;2019:7954921. doi: 10.1155/2019/7954921. [DOI] [PMC free article] [PubMed] [Google Scholar]