Figure 4. ZEB1 deficiency inhibits STAT3 phosphorylation during Th17 differentiation.
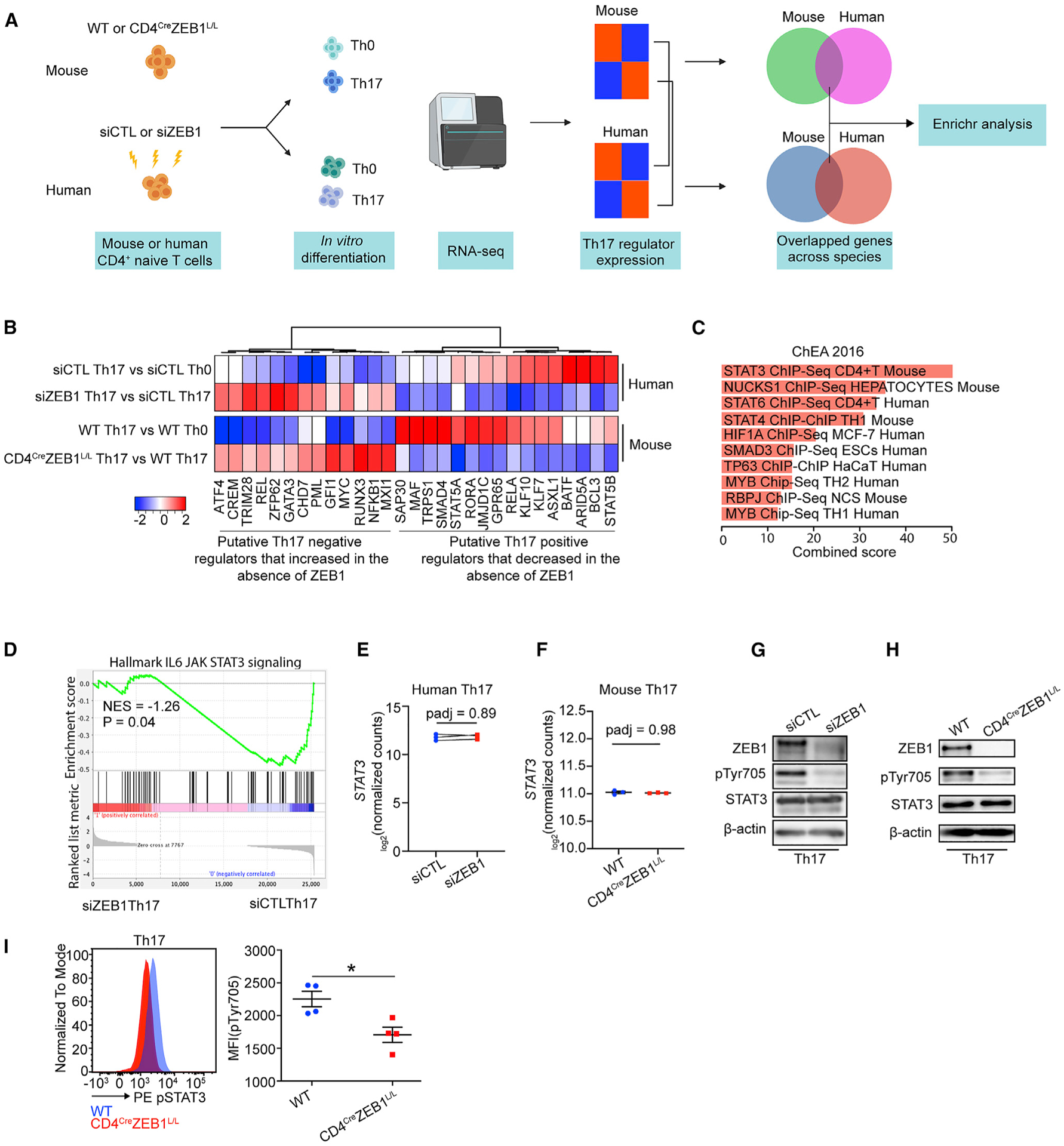
(A) Schematic of the experimental design to identify conserved effects of ZEB1 on both human and mouse Th17 transcriptional programs.
(B) Heatmap showing the expression changes of concordant ZEB1-dependent putative Th17 regulators in both human and mice.
(C) ChEA of conserved ZEB1-dependent Th17 regulator genes in (B) (p < 0.05).
(D) GSEA plot showing the effect of siZEB1 on JAK-STAT3 signaling in Th17 cells (p = 0.04). NES, normalized enrichment score.
(E and F) Transcription level of STAT3 in (E) human siCTL- and siZEB1-treated cells or (F) mouse WT and CD4CreZEB1L/L cells in Th17 condition.
(G and H) Western blots showing expression of total STAT3 and phospho-STAT3 (phosphorylation site: Tyr705) in human siCTL- or siZEB1-treated cells (G) and mouse WT or CD4CreZEB1L/L cells (H) under Th17 conditions.
(I) Flow cytometry results showing pTyr705 expression in mouse WT or CD4CreZEB1L/L cells under Th17 conditions.
A Representative result of at least two individual experiments is shown in (G) and (H). Symbols in (E) represent individual experiments. Symbols in (F) and (I) represents individual mice. The data in (F) and (I) are expressed as mean ± SEM. p values in (E) and (F) were calculated using Wald test and adjusted using the Benjamini-Hochberg method. p value in (I) was calculated using unpaired Student’s t test (two-tailed). *p < 0.05.
