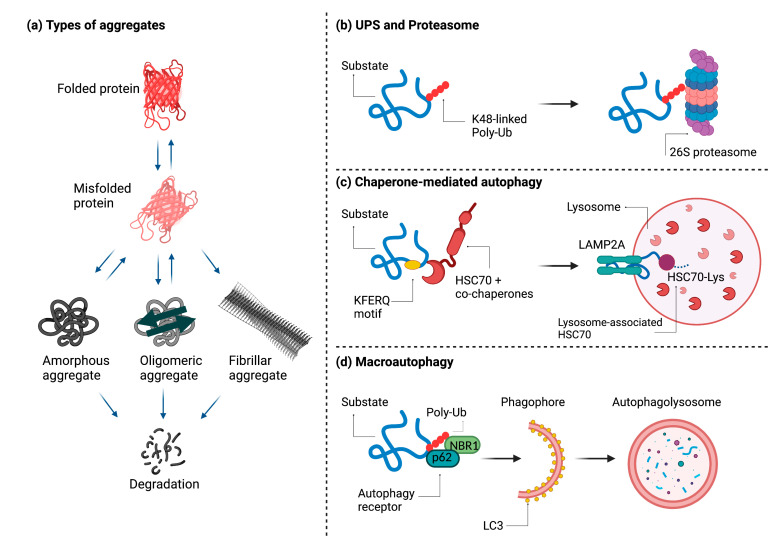
Figure 1.
Types of aggregates and degradation mechanisms. A functional protein is normally found in a properly folded three-dimensional state. Aggregation, which is normally prevented by molecular chaperones, can occur as a result of different stimuli and lead to the formation of three different types of aggregates: amorphous, oligomeric, or fibrillar. Except in the case of fibrillar aggregates, these proteins can return to their folded state. If this does not happen, they are degraded (a). Aggregates can be degraded in three different ways: by the ubiquitin-proteasome system (b), by chaperone-mediated autophagy (c), or by aggrephagy, a special form of macroautophagy (d). Figure created with BioRender.com (accessed on 3 August 2021).