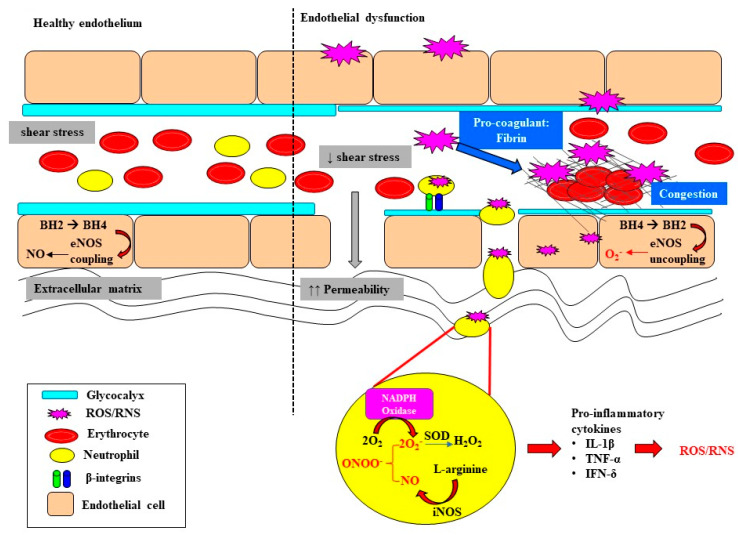
Figure 2.
A schematic outlining the initiation of oxidative damage to the endothelium, the downstream consequences for endothelial dysfunction and the propagation of oxidative stress. The layer of glycocalyx lining the apical surface of endothelial cells is important for the maintenance of shear-stress and flow-mediated nitric oxide (NO) release and vasodilation of the endothelium. Sepsis-induced oxidative stress leads to glycocalyx thinning and shedding, resulting in the loss of gap junctions, and subsequently increased permeability and vascular leakage. Loss of gap junctions also greatly facilitates extravasation of neutrophils in the circulation, mediated by β-integrins, through to the extracellular matrix. The resultant superoxide (O2−) that is formed can interact with nitrosative species generated by inducible nitric oxide synthase (iNOS), forming the highly reactive peroxynitrite (ONOO−) and then hydrogen peroxide (H2O2). Oxidative bursts from neutrophils contribute to enhanced oxidative damage and the downstream production of pro-inflammatory cytokines which have the intrinsic capability of producing reactive oxygen species (ROS) and reactive nitrogen species (RNS). Oxidative stress can also induce the recruitment of pro-coagulants to the site of injury, resulting in vascular congestion, ultimately impeding blood flow. Increased oxidative stress at the endothelium depletes and oxidizes the pool of tetrahydrobiopterin (BH4), resulting in the uncoupling of endothelial nitric oxide synthase (eNOS) in endothelial cells, thereby enhancing the production of ROS. Interleukin-1beta (IL-β); tumor necrosis factor-alpha (TNF-α), interferon-gamma (IFN-δ).