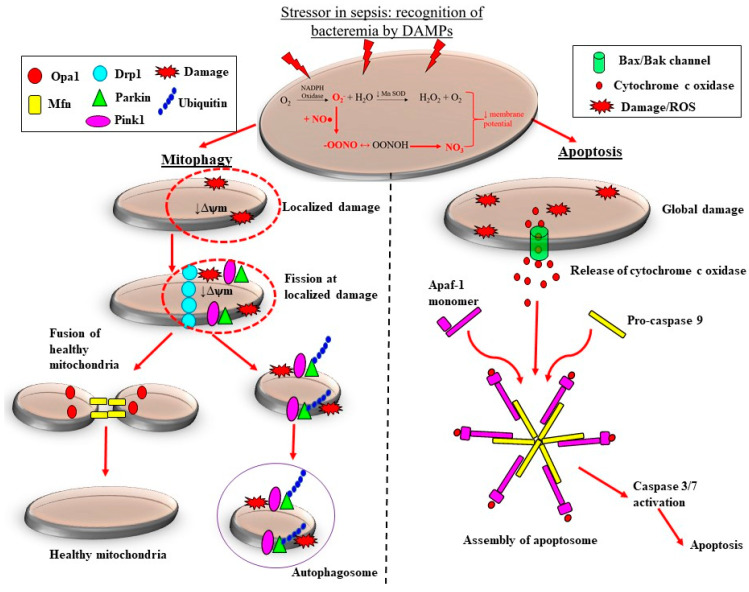
Figure 3.
Adaptive and maladaptive responses of the mitochondria to sepsis-induced oxidative stress. Increased production of superoxide (O2−) by nicotinamide adenine dinucleotide phosphate (NADPH) oxidase coupled with the reduced activity of manganese superoxide dismutase (MnSOD) results in the accumulation of O2−. Cytosolic nitric oxide (NO) produced by inducible nitric oxide synthase (iNOS) reacts with O2−, forming the highly reactive peroxynitrite (ONOO−). The accumulation of O2− and ONOO− results in persistent oxidative stress and a reduction in mitochondrial membrane potential (ψm) and so mitochondrial dysfunction. In the case of localized mitochondrial damage, mitochondrial quality control mechanisms are activated which arrest mitochondrial dysfunction and limit excessive mitochondrial loss. Recruitment of mitochondrial fission proteins to sites of injury targets the damaged portions of the mitochondrion for fission. Subsequently, ubiquitin, PTEN-induced kinase (PINK1) and E3 ubiquitin-protein ligase (Parkin) proteins are recruited to the damaged mitochondrion, removed by mitochondrial fission and interact with phagophore, consequently forming an autophagosome. The healthy portion of the mitochondrion undergoes mitochondrial fusion, adding to the existing mitochondrial pool and limiting excessive mitochondrial loss. On the other hand, extensive damage to mitochondria in severe sepsis results in the release of cytochrome c oxidase, and in the formation of an apoptosome when it interacts with apoptotic protease activating factor-1 (Apaf-1) monomers and pro-caspase 9. This leads to downstream activation of caspase 3/7, ultimately resulting in the containment of sepsis-induced damage via apoptosis. Mitochondrial dynamin-like GTPase (OPA-1); dynamin related protein-1 (DRP-1); damage-associated molecular patterns (DAMPs).