FIGURE 1.
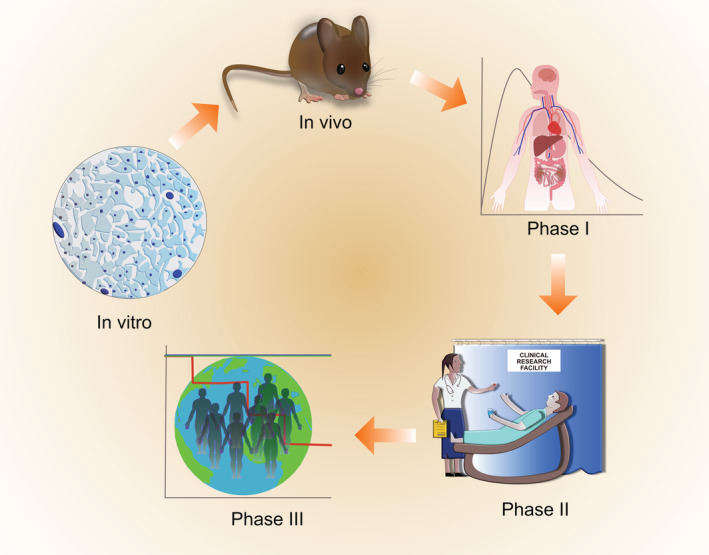
Translational pipeline. Candidate drugs are first investigated in vitro for example, in patient cell lines before in vivo toxicity and efficacy studies in appropriate animal models of disease are undertaken. Clinical trials include phase I studies, in which the candidate therapy is administered to patients or healthy volunteers to assess safety and tolerability, as well as drug pharmacokinetics. Phase II studies assess safety and efficacy of the drug in a small number of patients. Phase III studies assess safety and efficacy of the drug in a larger number of patients with defined outcome measures
