Figure 1.
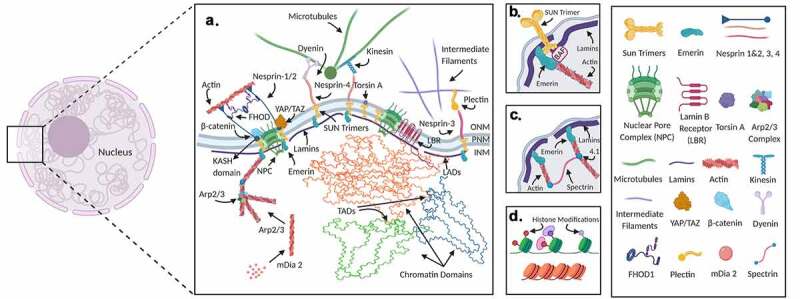
Nucleus is a mechanically integrated mechanosignaling center. Nuclear structural proteins interact with the cytoskeleton, chromatin, and the nuclear membrane to stabilize the nucleus and provide mechanosensing functions (Insert A). LINC complexes composed of Sun 1/2 trimers and Nesprin 1/2 mechanically couple the actin cytoskeleton. The LINC complex also interacts with nuclear pore complexes (NPC) and in-part regulate the access of important mechanical transducers such as β-catenin and YAP/TAZ into the nucleus. Nesprin-3 through interactions with plectin and nesprin-4 are also known to interact with cytoplasmic intermediate filaments and microtubules, respectively. Nesprins can also bind to microtubules via dynein and kinesin. Mechanical coupling of actin and the LINC complex involves cytoplasmic formins such as FHOD1 that attaches nesprins and actin at multiple points for a more robust association. Torsin A may also facilitate the LINC assembly at the nuclear envelope. A nuclear envelope transmembrane protein, Emerin connects the LINC complex, via SUN1/2 and nesprin-1/2 to the chromatin through BAF and lamin A/C (Insert B). Emerin also associates and plays a role in regulating extra and intranuclear actin. The intranuclear actin network is formed through the crosslinking of short F-actin fibers via protein 4.1 and spectrin that provides elastic structural properties to the nucleus (Insert C). Inside the nucleus, G-actin is assembled into linear and branched networks through regulatory proteins such as arp2/3 and mDia2 and influence chromatin dynamics and gene access. Chromatin domains that bind to the nuclear lamins are called lamin-associated-domains (LAD). These domains have been shown to be correlated with heterochromatin, producing repression of gene expression of genes in the LADs. These chromatin domains conserve epigenetic histone modifications. Changes of histone modifications, topologically associated domains (TADs), and LADs all result in changes in gene expression and cell differentiation (Insert D)
