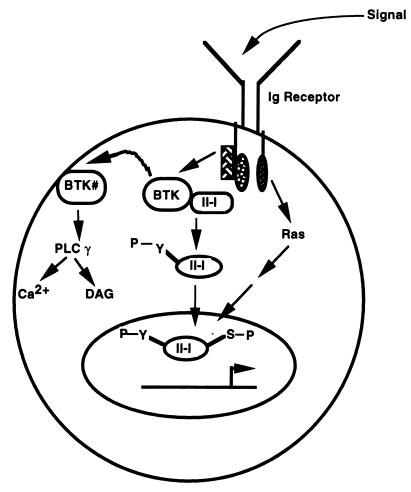
FIG. 8.
Model for Btk-dependent TFII-I function. A fraction of TFII-I is constitutively associated with Btk in the cytoplasm of wild-type resting B cells. Upon signaling through the Ig receptor, Btk is activated (Btk#), leading to tyrosine phosphorylation of TFII-I either directly or indirectly. While Btk# may localize to the plasma membrane to bind phospholipids, trigger calcium signaling, and activate diacyl glycerol (DAG), tyrosine-phosphorylated TFII-I is released from Btk# and translocates to the nucleus, where it is serine phosphorylated, perhaps through a Ras-dependent pathway. Although tyrosine phosphorylation of TFII-I may not be necessary for its nuclear import, it may be required for its maximal transcriptional activity. In xid B cells, TFII-I may not be constitutively associated with Btk and thus, increased amounts are in the nucleus.